INSTITUTIONAL ETHICS COMMITTEE
Institutional Ethics Committee – I (IEC-I)
Registered by : DCGI & Licensing Authority with Re-Registration
No.ECR/229/Inst/MH/2013/RR-19 issued under
NewDrugs and Clinical Trials Rules, 2019 and
the Drugs and Cosmetics Act, 1940.
Valid from April 22, 2019 thru April 21, 2024
Recognized by : The Strategic Initiative for Developing Capacity in
Ethical Review (SIDCER),Forum for Ethical committees in Asia and
the Western Pacific Region (FERCAP) for its compliance with
international and local standards in ethical review.
Accredited by : National Accreditation Board for Hospitals (NABH) &
Healthcare Providers (Constituent Board of Quality Council of
India).
Valid from May 19, 2021 thru May 18, 2024 Certificate No. EC-
CT- 2018-0027
Institutional Ethics Committee – II (Relating to Biomedical Health Research)
Registered By: Biomedical and Health Research (NECRBHR),
Department of Health Research (DHR).
Recognized by: The Strategic Initiative for Developing Capacity in Ethical
Review (SIDCER), Forum for Ethical committees in Asia and
the Western Pacific Region (FERCAP) for its compliance with
international and local standards in ethical review.
Institutional Ethics Committee – III (Relating to Biomedical Health Research.)
Registered By: Biomedical and Health Research (NECRBHR),
4.IEC Protocol review processing fees
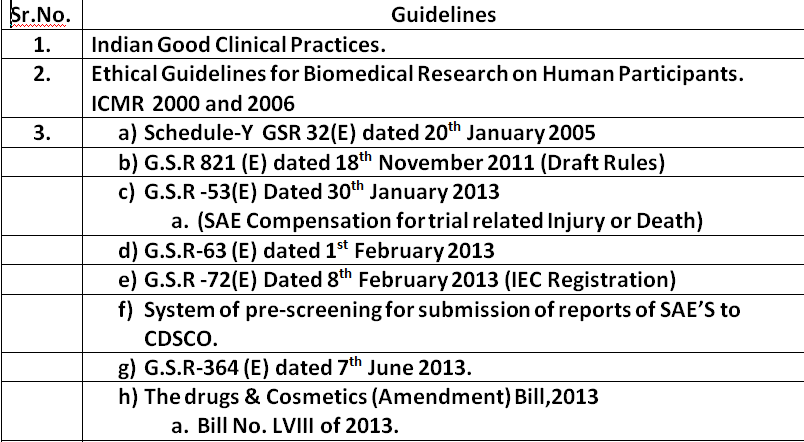
6. Standard operating procedures (SOP 1 to 22) V6.1 dated 29th June 2020.
8.SOP 24 Version 1 (Review of Case Report and Case Series)
9.SOP 25 Version 1 Audio Visual (AV) Recording of Informed Consent Process
11. Member list
- Member List of IEC-I (Tenure 8th December 2022 to 16th October 2025)
- Member list of IEC-II (Tenure 22nd December 2022 to 16th October 2025)
- Member List of IEC III (Tenure 22nd Dec 2022 to 16th Oct 2025)
- CDSCO letter of approved New IEC- Composition dated 18th November 2022
- DHR New Composition of IEC I Tenure 2022-2025
- DHR New Composition of IEC II Tenure 2022-2025
- DHR New Composition of IEC III Tenure 2022-2025
12.Notice – CDSCO Dated 25-02-2021 for SAE reporting
- SAE_UserManual _(Online _&_Offline)
- SAE Reporting For Offline CT
- CDSCO NOTICE Dated 25-02-2021 for SAE reporting
13.Circular dated 21st March 2023 regarding protocol submission
14.Important circular for Investigators regarding Submissions
A. Annexures for initial submission
B. Vulnerable Group Annexures
C. Other Annexures
D. SAE Annexures
Click here to view complete table
| IEC-I | IEC-II | IEC-III | |
| Member Secretary | Dr. Raakhi Tripathi
Associate Professor Pharmacology & Therapeutics Intercom no. 7444,7482 Mobile no. 9821724700 |
Dr. Priyanka Prasad
Associate Professor Microbiology Intercom no. 7552 Mobile no. 9930923115 |
Dr. Swapna Kanade
Associate Professor Microbiology Intercom no. 7827 Mobile no. 9004377725 |
| Jt. Member Secretary | Dr. Nithya Gogtay
Prof. and Head, Clinical Pharmacology Intercom no. 7715 Mobile no. 9820495836 |
Dr. Mona Agnihotri
Associate Professor (Additional) Pathology Intercom no. 7566, 7555 Mobile no. 9987810111 |
Dr. Shirish Joshi
Professor (Additional) Pharmacology & Therapeutics Intercom no. 7444 Mobile no. 9820338456 |
Office Address:
Institutional Ethics Committee (IEC) I, II & III
New UG-PG Hostel, 20 Storey Hostel Building, Ground Floor,Seth G.S. Medical College & K.E.M. Hospital, Parel, Mumbai 400 012.
Telephone no: 2412 2188, 24107515
Intercom no. 7515
E-mail: iec-1@kem.edu
E-mail: iec-2@kem.edu
E-mail: iec-3@kem.edu
The IEC Office timing for correspondence and communication is as follows: The office hours for submission of documents, enquiries and telephonic communication with the IEC staff are as follows:
Monday to Friday – 1.30 p.m. to 4.30 p.m.
Saturday – 10.30 a.m. to 12.30 p.m.
- The office will remain closed on Sundays and all the public holidays.
- The IEC office remains closed for the last working day of every month for monthly data update of IECs.
- You are requested to follow the office timings strictly for submission and telephonic enquiries and kindly cooperate with the IEC staff.
News and Updates:
- Notice:This is regarding the IECs approval of dissertations topics of MD (Pediatrics) postgraduate students of Seth GSMC & KEMH. Kindly note it has come to IECs notice that dissertations from BJJWHC are being directly sent to IEC without them being forwarded by HOD (Pediatrics) Seth GSMC.
- Institutional Ethics Committee Circular
For Investigators:
2. Sample format Cover Letter for Pharmaceutical & GOVT sponsored Funded studies
3. For online GCP training certification follow website link : gcp.nihtraining.com
4.Sample format of Memorandum of Understanding(MOU)
5.Circular : who should be the Principal investigator
6.Revised sample format of DRB Approval letter Dated 21st March 2023
7.Revised Departmental Review Board Guidance Document dated 21st March 2023

 Users Today : 36
Users Today : 36 Total Users : 138314
Total Users : 138314 Who's Online : 1
Who's Online : 1