
KING EDWARD MEMORIAL HOSPITAL
SETH GORDHANDAS SUNDERDAS MEDICAL COLLEGE
बृहन्मुंबई महानगरपालिका रुग्णालय


KING EDWARD MEMORIAL HOSPITAL
SETH GORDHANDAS SUNDERDAS MEDICAL COLLEGE
बृहन्मुंबई महानगरपालिका रुग्णालय


The radiology department is one of the largest teaching departments in India both in terms of clinical services provided and the radiology residency training program. The department examines over 160,000 patients each year. All forms of diagnostic and interventional radiological procedures including sophisticated vascular and neuro interventional procedures are performed. The department has a very active teaching schedule for residents besides conducting refresher courses; it also provides opportunity for Observer ship for those from other departments and elsewhere in the state and country. The department boasts one of the best departmental book libraries in the country with over 800 volumes and an exhaustive film and electronic teaching media library.
We welcome all our alumni to this meeting ground and sincerely hope that this site will help you to renew your ties with The Department. You may have been here at these institutions just a few years ago or a few decades ago – but all of us are from the same breed – Radiologists trained at KEM.
Since your times…whenever it was…things have changed!! and those of still here relish our days past, look forward to the days in future striving at all times to keep the department flag flying high. To be able to do this we need your support in…many ways…but in one most important way…please keep in touch. That is one of the very reasons this website is there so we can interact with our alumni…all over the world…and use their suggestions and inputs to make this department stand apart from the rest. I am sure in the true GS tradition most of us will be interested in the Alumni activities not as much ‘to get’ but much more ‘to give’ to our Alma Mater.
We urge you to become members of the G.S. Alumni Association…submitting this form. For those of you who need and can, membership of the GOSUMEC Alumni association entails you to the free use of the College Library which is reasonably well stocked with books and journals. Besides, you will be delighted to know that we have setup an electronic teaching library of all modalities in radiology in the department. You may want to visit and use it.
We now come to the ‘giving’ part. There is a lot each of us individually and collectively do to help the department. As times have gone by the funding to the department’s teaching and clinical activities has become precarious with help being need in several areas both in cash and kind. You can contribute to this and all help in whatever form will be welcome. If you wish to get involved please drop in a line at this address websitecontact@kem.edu with what you have in mind and we could start working on it.
And finally…if you are anywhere near Parel… or passing by, please take time out to drop in the department even if only Deshi and the techs and the good old ward boys are the only ones who will recognize you!!!! Come have a cup of Tea at the KEM Canteen Katta…
“Please take a moment to let us know you have been here by filling this form so that we can keep in touch”. We soon hope to be able to develop a database of all our alumni so that you can communicate with your long lost friends.
Case of the Month – Jan 2006
Contributed by Dr. Nilesh Porwal
CLINICAL PROFILE :
On physical examination, there was mild icterus with tender hepatomegaly. However no lymphadenopathy was found.
Radiological findings:
Ultrasonography of the abdomen showed multiple, well defined hypoehoeic lesions – approximately 3cm in diameter – spread through out the liver with distortion of its contour.
Fig 1.
Plain and contrast enhanced CT scans of the abdomen revealed multiple, target lesions with minimal rim enhancement, hepatomegaly, free fluid in peritoneal cavity & enlarged aorto-caval lymph nodes.
Fig 2
Fig 3.
On MRI, these lesions appear as hyperintense on both T1 & T2 weighted images. This ruled out the possibility of fungal abscesses which are hypointense on T2 as it contain manganese & iron.
Fig 4.
Fig 5.
Fig 6.
DISCUSSION: –
There are three main categories of AIDS related lymphoma.
1.Imunoblastic lymphomas {60%}: High grade or diffuse histocytic lymphoma.{large cell} common in older patients.
DIFFERENTIAL DIAGNOSIS:-Lesions due to metastases, Kaposi’s sarcoma, disseminated tuberculosis, bacillary angiomatosis & Pneumocystis carinii may present similar appearances.
INCIDENCE:- Non Hodgkin Lymphoma is present in 3% of HIV positive patients at the time of their diagnosis & develops in upto 20 % HIV patients during their life time.
CLINICAL PRESENTATION: – The most common symptom is painless swelling of lymph nodes in the neck, axilla and groin. 80% of patients present with advanced disease/extra nodal presentation; of these, 80% have type B symptoms at presentation like unexplained fever, night sweats, fatigue, weight loss & anorexia.
TYPE OF NHL: – Low grade, intermediate& high grade lymphoma depend upon microscopic appearance.
DIAGNOSIS :- Biopsy is necessary to confirm the diagnosis. Other lab parameters & imaging modalities are helpful to monitor & determine the spread of disease.
STAGING OF DISEASE:- Stage I: Disease located to one region; Stage II: located to two regions on the same side of the diaphragm& Stage III: Spread to both sides of the diaphragm involving one organ or area near-by spleen or lymph nodes. In stage IV, spread beyond lymphatic system, involving one or more major organ possibly bone marrow, skin.
TRATMENT OPTION :- 1} Combination of chemotherapy
2}Radiation therapy usually along with chemotherapy.
3}Bone marrow transplant in case of recurrence.
4}Immunotherapy.
In a seropositive patient these are usually combined with antiretroviral therapy.
CONCLUSION: – AIDS Clinical trial group study suggested that prognostic variables in AIDS patients closely resemble with that of non-AIDS related NHL.
Two year survival for patients with good prognosis treated with chemotherapy is 50 % as compared with 24 % for those with poor prognosis.
Academic achievements:
More than 150 scientific published papers in the last ten years in
The journal of neurosurgery [USA],
British journal of neurosurgery,
Acta neurochirurgie,
Neuromedchirur[Tokyo],
Clinical neurosciences[Australia]
Most active academic neurosurgical unit in India
Workshop
Department of Neurosurgery, KEM Hospital had held National level Conference recently for first time in history of Neurosurgery not only in country but also in world.
7 Workstations which were held are given below-
Following workstation, extensive live demonstration of Neurosurgical technique in 6 cases were shown on single day. Academic feast was arranged by the faculty and preceded by young Neurosurgeon Best Award Paper.
This is also a first time tribute to a teacher Dr.(Prof) B. S. Das from Orissa, which was expressed as a theme of the meeting
Case of the month April 2006
Case contributed by Dr. Girish Yenvankar
Clinical profile:
A 29-yeaar-old lady presented with dysphagia since two years. The dysphagia was more for
solids than liquids and was progressively increasing. There was no history of vomiting, weight
loss or fever.
Radiological Findings –A plain radiograph of the chest showed a soft tissue opacity in the right
paravertebral region with an air-fluid level in the upper dorsal region This suggested a dilated
esophagus(Fig 1).
Fig 1
A CT scan of the chest confirmed this. There was narrowing at the lower end of the esophagus.
No soft tissue mass was seen in the region of the narrowing (Figs 2,3,4)
Fig 2,3,4
A barium esophagogram a showed smooth narrowing at the gastroesophageal junction
extending for a distance of about 2 cms. with moderate dilatation of the proximal esophagus. A
bird-beak appearance is seen at the distal portion of the esophagus with no evidence of
shouldering. The esophageal mucosal pattern was normal. It showed no intrinsic or extrinsic
filling defects or mass effect. Tertiary contractions were seen in the proximal esophagus.
There was no evidence of hiatal hernia or gastro-esophageal reflux.
Figs 5,6,7,8
The patient was diagnosed to have achalasia cardia.
Discussion:
Dysphagia is the most common presenting symptom in patients with achalasia. The ingestion of either solids or liquids can result in dysphagia, though dysphagia for solids is more common. The
natural history varies. Some patients notice that the dysphagia reaches a certain point of severity and then stops progressing. In others, the dysphagia continues to worsen, resulting in decreased oral intake and malnutrition. Therefore, weight loss is included in the complex of signs and symptoms associated with achalasia, and it is usually a sign of advanced esophageal disease. Some of patients with dysphagia complain of episodes of chest pain which are frequently induced by eating. Typically, chest pain is described as being retrosternal; this is a more common feature
in patients with early or so-called vigorous achalasia. As the disease progresses and as the esophageal musculature fails, chest pain tends to abate or disappear.
Many of patients with achalasia experience spontaneous regurgitation of undigested food from
the esophagus during the course of the disease. Some learn to induce regurgitation to relieve the
retrosternal discomfort related to the distended esophagus. As the disease progresses the likelihood that aspiration will occur increases. As a result, some patients may present with signs or symptoms of pneumonia or pneumonitis. Lung abscesses, bronchiectasis, and hemoptysis are some of the more severe pulmonary consequences of
achalasia-associated aspiration.
Pathophysiology: The exact etiology of achalasia is not known. The most widely accepted current theories implicate autoimmune disorders, infectious diseases, or both. The last decade has witnessed much progress in the understanding of the cellular and molecular derangements in achalasia. Degeneration of the esophageal myenteric plexus of Auerbach is the primary histologic finding.
Radiologcial Studies
Plain radiograph –
Findings:
Plain chest radiographs occasionally offer clues in the diagnosis of achalasia. A double mediastinal stripe is occasionally depicted. An air-fluid level can be seen in the esophagus; this is frequently retrocardiac. Owing to the paucity of air progressing through the hypertensive LES, the gastric air bubble may be small or absent.
Barium Swallow-
Features of achalasia depicted at barium study under fluoroscopic guidance include the following:
Failure of peristalsis to clear the esophagus of barium with the patient in the recumbent position
Antegrade and retrograde motion of barium in the esophagus secondary to uncoordinated,
nonpropulsive, tertiary contractions Pooling or stasis of barium in the esophagus when the esophagus has become atonic or noncontractile (which occurs late in the course of disease)
LES relaxation that is incomplete and not coordinated with esophageal contraction
Dilation of the esophageal body, which is typically maximal in the distal esophagusn Tapering of the barium column at the unrelaxed LES, resulting in the bird beak sign Associated epiphrenic diverticula sometimes seen.
CT scan –
CT scanning with oral contrast enhancement may demonstrate the gross structural esophageal abnormalities associated with achalasia, especially dilatation, which is seen in advanced stages. However, CT findings are nonspecific, and the diagnosis of achalasia cannot be made using CT alone. CT scan may be indicated in the workup of patients with suspected pseudoachalasia.
Treatment: .
Pharmacologic therapy for achalasia: Calcium channel blockers – Nifedipine and verapamil ,Anticholinergic agents – Cimetropium bromide ,Nitrates – Isosorbide dinitrate ,Opioids – Loperamide
Pneumatic Balloon Dilatation -Mechanical therapy for achalasia consists of esophageal dilation,
the object of which is to disrupt muscle fibers of the LES, effecting a decrease in LES pressure.
Dilation is most commonly performed by using pneumatic balloons.
Botulinum toxin (Botox) therapy –This is new modality of treatment
Esophageal (Heller) myotomy is a surgical procedure that is now commonly performed with
minimally invasive techniques. The laparoscopic approach appears to be most appropriate.
CASE OF THE MONTH MARCH 2006
Contributed by Dr. Yogeshwari Deshmukh
Clinical history
A 22-year-old man presented with fullness of abdomen associated with intermittent episodes of vomiting since six months. Except for his asthenic built, there was no positive physical finding on examination. Routine laboratory investigations were within normal limits.
Radiological examination:
An upper GI series revealed dilatation of the first and second parts of the duodenum with an abrupt vertical cut off at its third part. The mucosal pattern was normal. The obstruction to passage of barium was dramatically relieved in the lateral decubitus position
Fig.1, 2
A contract enhanced CT scan of the abdomen showed a dilated proximal duodenum with abrupt cut off at its third part. The superior mesenteric artery (SMA) was seen to pass anterior to the site of the cut off. This was associated with a narrowed aorta-SMA distance.
These radiological findings were interpreted as a manifestation of the superior mesenteric artery syndrome.
The patient was operated. Intraoperative findings confirmed the diagnosis of SMA syndrome as the third part of duodenum was seen to be compressed between the aorta and SMA. A Roux-en-Y jejunostomy was performed.
Discussion:
The SMA syndrome is an uncommon, but well recognized, clinical entity characterized by compression of the third or transverse portion of the duodenum against the aorta by the SMA – resulting in chronic, intermittent or acute – complete or partial duodenal obstruction. The SMA syndrome was first described in 1861 by Von Rokitansky, who
proposed that its cause was obstruction of the third part of the duodenum as a result of arteriomesenteric compression.
Clinical Presentation:
Patients often present with chronic upper abdominal symptoms such as epigastric pain, nausea, eructation, voluminous vomiting (bilious or partially digested food), postprandial discomfort, early satiety, and sometimes – sub acute small-bowel obstruction. The symptoms are typically relieved when the patient is in the left lateral decubitus, prone or knee-to-chest position and they are often aggravated when the patient is in the supine position. An asthenic habitus is noted in about 80% of cases. Abdominal examination may reveal a succussion splash.
Pathophysiology:
The SMA usually forms an angle of approximately 45° (range, 38-56°) with the abdominal aorta. The third part of the duodenum crosses caudal to the origin of the SMA coursing between the SMA and the aorta. Any factor that sharply narrows the aortomesenteric angle to approximately 6-25° can cause entrapment and compression of
the third part of the duodenum as it passes between the SMA and the aorta – resulting in the SMA syndrome. In addition, the aortomesenteric distance in SMA syndrome is decreased to 2-8 mm (normal is 10-20 mm). Alternatively, other causes implicated in the SMA syndrome include high insertion of the duodenum at the ligament of Treitz, a low origin of the SMA and compression of the duodenum due to peritoneal adhesions. The important etiologic factors that may precipitate a narrowing of the aortomesenteric angle and result in chronically recurrent mechanical obstruction include the following:
Constitutional factors
Thin body build
Exaggerated lumbar lordosis
Visceroptosis and abdominal wall laxity
Depletion of the mesenteric fat caused by rapid severe weight loss due to
catabolic states such as cancer and burns
Severe injuries, such as head trauma, leading to prolonged bed rest
Dietary disorders: Anorexia nervosa, Malabsorption
Spinal disease, deformity, or trauma
Use of body cast in the treatment of scoliosis or vertebral fractures
Anatomic anomalies –
Abnormally high and fixed position of the ligament of Treitz with an upward
displacement of the duodenum
Unusually low origin of the SMA
Imaging Studies:
The diagnosis of SMA syndrome is difficult. Confirmation usually requires radiographic studies such as an upper GI series, hypotonic duodenography and CT scanning.
An uipper GI study with barium reveals characteristic dilatation of the first and second parts of the duodenum with an abrupt vertical or linear cutoff in the third part with normal mucosal folds. Fluoroscopy demonstrates a to-and-fro motion of the barium in the dilated proximal portion of the duodenum. Other findings include a delay of 4-6 hours in gastroduodenal transit and relief of the obstruction when the patient is in the left lateral decubitus position. A Hayes maneuver (i.e. pressure applied below the umbilicus in cephalad and dorsal direction), which elevates the root of small-bowel mesentery may also relieve the obstruction.
CT scanning is useful in the diagnosis of the SMA syndrome and can provide diagnostic information including the aorta-SMA distances and duodenal distension. Also, it can be used to assess intra-abdominal and retroperitoneal fat.
Upper GI endoscopy may be necessary to exclude mechanical causes of duodenal obstruction. However, the diagnosis of SMA syndrome may be missed with this study.
Abdominal Ultrasonography may be helpful in measuring the angle of the SMA and the aortomesenteric distance.
Treatment
Medical Care: Reversing or removing the precipitating factor is usually successful in a patient with acute SMA syndrome. Conservative initial treatment is recommended in all patients with the SMA syndrome; this includes adequate nutrition, GI decompression, and proper positioning of the patient after eating (i.e. left lateral decubitus, prone, or knee-to-chest position).
Surgical Care: Surgical intervention is indicated when conservative measures are ineffective, particularly in patients with a long history of progressive weight loss, pronounced duodenal dilatation with stasis and complicating peptic ulcer disease. Duodenojejunostomy is the most frequently used procedure and it is successful in about 90% of cases. The use of laparoscopic surgery that involves lysis of the ligament of Treitz and mobilization of the duodenum has also been reported.
February 2006
Case contributed by Dr.Manish Shinde
A seven-year-old girl presented with complaints of fever and joint pains since one month. She gave history of vague abdominal pain since one month. Since then too, there was a progressively increasing swelling on the vertex of the skull. On examination, this swelling had a firm consistency and was not well defined. The patient’s vital parameters were normal.
RADIOLOGICAL EXAMINATION
A radiograph of the abdomen was unremarkable.
Ultrasonography of the abdomen revealed a well-defined, hypoechoic mass measuring 6x4x4 cms in the left adrenal fossa on the upper pole of the left kidney displacing the kidney inferiorly. It was hypo-vascular with fine calcification within.
Fig 1
Fig2
A plain and contrast enhanced CT scan of the abdomen revealed a well defined 6x4x4 cms hypo dense, hypo vascular mass lesion involving the left adrenal gland with displacement of the kidney inferiorly and laterally. No surrounding infiltration was noted. Fine calcification was seen within the mass.
Fig 3
Fig 4
The skull film showed a destructive lesion with a sunray periosteal reaction over the left parietal bone.
Fig 5
Fig 6
Sonography of the skull confirmed the sunray periosteal reaction and showed a subgaleal hypoechoic soft tissue mass measuring 11x9x 8 cm.
Fig 7
A CT scan of the skull revealed lytic lesions in the left parietal bone with sunray periosteal reaction with subgaleal mass with underlying dural involvement suggestive of a metastatic involvement of the calvarium and dura.
Fig 9
A bone marrow smear was performed which showed atypical round cells suggestive of a neural crest tumour metastases.
MIBG scan showed increased I131 MIBG concentration within marrow cavity of the skull and spine indicating metastases.
Bone scan revealed metastases to the skull and spine.
Final diagnosis – Left neuroblastoma with metastases. Stage 4 INSS staging.
Discussion –
Neuroblastomas are the commonest extracranial tumour in children and account for 6-8 % of pediatric malignancies. They originate from the cells of the neural crest origin which give rise to the sympathetic nervous system and adrenal medulla. The median age of patient at the time of diagnosis is two years but the tumour can present at any pediatric age.
Two thirds of patients with neuroblastoma have metastasis (Stage IV disease) at the time of presentation. Metastasis is commonly to the liver, spine, skull and lymph nodes. Staging is done based on the International Neuroblastoma Staging System (INSS) from stage 1 to 4S based on radiological findings, surgical resectability, lymph node involvement and bone marrow involvement.
Technetium 99m methylene diphosphonate whole body bone scintigraphy is a must for detection of metastases. MIBG scan, though less sensitive than MDP should also be done as it can detect both the primary and metastases. FDG PET is likely to play a larger role in neuroblastoma imaging in future.
All patients older than one year with stage IV tumors are considered to be in the high-risk group. These patients seem to require treatment with multi-agent chemotherapy, surgery, and radiotherapy followed by consolidation with high-dose chemotherapy and peripheral blood stem cell rescue.
The five year survival rate from diagnosis is approximately 83% for infants, 55% for children aged 1-5 years, and 40% for children older than 5 years
Case of the Month – Jan 2006
Contributed by Dr. Nilesh Porwal
CLINICAL PROFILE :
A 35-year-old man, a known seropositive patient, symptomatic since one month came with complaints of dull aching, non radiating pain in the right hypochondrium & in the epigastric region associated with intermittent fever. The patient had history of pulmonary tuberculosis 15 yrs back & was on antiretroviral therapy since 1 ½ years.
On physical examination, there was mild icterus with tender hepatomegaly. However no lymphadenopathy was found.
Radiological findings:
Ultrasonography of the abdomen showed multiple, well defined hypoehoeic lesions – approximately 3cm in diameter – spread through out the liver with distortion of its contour.
Fig 1.
Plain and contrast enhanced CT scans of the abdomen revealed multiple, target lesions with minimal rim enhancement, hepatomegaly, free fluid in peritoneal cavity & enlarged aorto-caval lymph nodes.
Fig 2
>On MRI, these lesions appear as hyperintense on both T1 & T2 weighted images. This ruled out the possibility of fungal abscesses which are hypointense on T2 as it contain manganese & iron.
Fig 4.
On liver biopsy, the liver was heavily infiltrated by atypical looking mononuclear cells. There was biliary ductular proliferation. Very little normal liver parenchyma is seen. Some tumor cells showed nuclear moulding. These findings were highly suggestive of a Non Hodgkin’s Lymphoma.
Fig 6.
DISCUSSION: –
There are three main categories of AIDS related lymphoma.
1.Imunoblastic lymphomas {60%}: High grade or diffuse histocytic lymphoma.{large cell} common in older patients.
DIFFERENTIAL DIAGNOSIS:-Lesions due to metastases, Kaposi’s sarcoma, disseminated tuberculosis, bacillary angiomatosis & Pneumocystis carinii may present similar appearances.
INCIDENCE:- Non Hodgkin Lymphoma is present in 3% of HIV positive patients at the time of their diagnosis & develops in upto 20 % HIV patients during their life time.
CLINICAL PRESENTATION: – The most common symptom is painless swelling of lymph nodes in the neck, axilla and groin. 80% of patients present with advanced disease/extra nodal presentation; of these, 80% have type B symptoms at presentation like unexplained fever, night sweats, fatigue, weight loss & anorexia.
TYPE OF NHL: – Low grade, intermediate& high grade lymphoma depend upon microscopic appearance.
DIAGNOSIS :- Biopsy is necessary to confirm the diagnosis. Other lab parameters & imaging modalities are helpful to monitor & determine the spread of disease.
STAGING OF DISEASE:- Stage I: Disease located to one region; Stage II: located to two regions on the same side of the diaphragm& Stage III: Spread to both sides of the diaphragm involving one organ or area near-by spleen or lymph nodes. In stage IV, spread beyond lymphatic system, involving one or more major organ possibly bone marrow, skin.
TRATMENT OPTION :- 1} Combination of chemotherapy
2}Radiation therapy usually along with chemotherapy.
3}Bone marrow transplant in case of recurrence.
4}Immunotherapy.
In a seropositive patient these are usually combined with antiretroviral therapy.
CONCLUSION: – AIDS Clinical trial group study suggested that prognostic variables in AIDS patients closely resemble with that of non-AIDS related NHL.
Two year survival for patients with good prognosis treated with chemotherapy is 50 % as compared with 24 % for those with poor prognosis.
Case of the Month – May 2009
Case contributed by Dr. Rahul Shinde.
CLINICAL PROFILE:
A 8-year-old boy presented with multiple bony hard swellings over the para spinal region, gradually increasing in size over a two year period with progressive limitation of movements. At present, the child is barely able to flex and can minimally rotate his head.
The child was born out of non consanguineous marriage, full term normal delivery with no ante, intra or postnatal complications. Family history was not significant.
His symptoms started around two years ago, when he developed swellings over the para spinal region which were soft in consistency. There was no associated pain or fever. He was investigated with multiple x rays.
Figure 1
Figure 2
The radiographs revealed no abnormality except for congenital fusion of C 4-5-6 vertebrae. The swelling subsided on its own.
About eight months ago, he developed a similar swelling in the same region. To investigate this swelling, an MRI of the spine spine and biopsy was done.
Figure 3
Figure 4
MR spine revealed extensive T 2 hyperintense signal in bilateral para spinal muscles (edema) without any spinal abnormality suggesting myositis.
Biopsy of the para spinal muscles revealed inflammatory changes in the muscle with minimal fibrosis. Impression was Inflammatory Myositis vs Duchene muscular dystrophy.
The child progressively worsened with increasing limitation of movements of the cervical and lumbar regions and was referred to our institution for further management.
On physical examination, multiple bony hard swellings about 4-6 cms in diameter were noted in the cervical and dorsolumbar region. The lesions were fixed. There was no tenderness, warmth, redness or any evidence of inflammation. The patient had no other bony abnormality except for hallux valgus. The range of movement at the cervical and dorso lumbar spine was severely restricted.
CURRENT INVESTIGATIONS:
Radiographs of the cervical and dorso lumbar spine were taken.
Figure 5
Lateral radiograph of the cervical spine reveals congenital fusion of C 4-5-6 vertebrae with decreased intervertebral space. There is linear sheet like calcification seen just beneath the subcutaneous plane extending inferiorly from the sub occipital region.
Figure 6
Lateral radiograph of the dorso lumbar region shows similar sheet like of calcification in the dorso lumbar region just beneath the subcutaneous plane.
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
MR scan of the cervical region reveals multiple hypointensities in both T1 and T2 weighted sequences in both para spinal muscles suggestive of calcification. Subtle T2 hyperintense signals are seen in the paraspinal muscles (edema) suggesting myositis.
Based on the symptomatology and imaging findings, diagnosis of Fibrodysplasia ossificans progressiva was made.
DISCUSSION:
Myositis ossificans progressiva (also known as fibrodysplasia ossificans progressiva) is a severe, rare condition of ectopic
ossification with primary involvement of the skeletal muscles, associated with characteristic skeletal abnormalities.
Genetics:
It has autosomal dominant inheritance with complete penetrance but variable expressivity, and most cases result from a sporadic mutation. Genes for bone morphogenetic proteins, in particular BMP4, are thought to be plausible candidate genes. Mutations in the ACVR1 gene cause fibrodysplasia ossificans progressive.
The ACVR1 gene provides instructions for producing a member of a protein family called bone morphogenetic protein (BMP) type I receptors. The ACVR1 protein is found in many tissues of the body including skeletal muscle and cartilage. It helps to control the growth and development of the bones and muscles, including the gradual replacement of cartilage by bone (ossification) that occurs in normal skeletal maturation from birth to young adulthood.
Researchers believe that a mutation in the ACVR1 gene may change the shape of the receptor under certain conditions and disrupt mechanisms that control the receptor’s activity. As a result, the receptor may be constantly turned on (constitutive activation). Constitutive activation of the receptor causes overgrowth of bone and cartilage and fusion of joints, resulting in the signs and symptoms of fibrodysplasia ossificans progressiva.
Clinical Course:
Ectopic ossification usually starts in early childhood. Radiological
evidence of ossification is not usually present until 4–6 weeks after the appearance of a lump. Sites of ossification include the neck, spine and shoulder girdle. Trauma precipitates new lesions. The ESR may be elevated during an acute episode. Radiological abnormalities of the toes and thumbs are common (Hallux valgus as in our case).
Imaging Studies:
Histopathology : Histologically, early lesions resemble granulation tissue, occasionally with cellular inflammatory infiltrate. Spicules of bone appear in the centre of the fibroblastic nodules. Bone and cartilage formation is seen in mature specimens. The bone formed initially is of the woven type; this is later remodelled to mature lamellar bone.
Management : There is little convincing evidence that any form of treatment alters the progress of the disease in myositis ossificans progressiva. Treatments that have been used include a diet reduced in vitamin D and calcium, the avoidance of sunshine, and treatment with corticosteroids. Other treatment strategies include beryllium, vitamins B and E and penicillamine. Drugs that block calcification have also been used, including EDTA (disodium ethylene diamine tetraacetic acid), isotretinoin and etidronate , without proven benefit.
Case of the month – October 2007
Case contributed by Dr. Mukta Agrawal
Clinical profile
A 13-months-old male child presented with gradual distention of abdomen over a period of 25 days with fever and cough since four days. On physical examination, the patient was afebrile and anicteric. There was fullness in the right upper quadrant of the abdomen due to hepatomegaly . No other significant finding was detected on physical examination.
Laboratory investigation: SGPT- 22U/L, SGOT-42U/L, Total bilirubin- 0.54mg%, BUN-
11mg%, Hb- 9.3gm%, Serum Alfa fetoprotein- 9.81ng/ml ( normal range- 0 to 13.4 ng/ml), Beta
HCG-absent.
Radiological findings
An ultrasound examination of the abdomen (Figs 1,2) revealed a large 10.5 x 9.5 x 10 cm sized anechoic cystic mass occupying almost the entire right lobe of the liver. There were multiple internal septations within. However, no solid component or vascularity within it was seen. The left lobe of the liver was enlarged. The portal vein showed normal flow; its right branch splayed over it anterosuperiorly. No portal or retroperitoneal lymphadenopathy was seen
nor was there ascitis. The rest of the abdomino-pelvic ultrasound examination was normal.
Figs. 1, 2
A plain and contrast enhanced CT of the abdomen (Figs. 3,4,5) confirmed the USG findings and showed a large well-defined cystic mass with few internal septations in the right lobe of liver measuring approximately 10 x 8 x 13cms occupying segments VII , VIII and VI. There was no calcification, nor was there any soft tissue component with in. On contrast scan, the septae showed enhancement.
Figs. 3,4,5
The mass was seen to displace the middle hepatic vein; the right hepatic vein was not visualised and the left hepatic vein was normal. The transhepatic IVC was compressed. The mass was abutting the portal vein bifurcation. The main portal vein at the porta and its left branch were normal. The anterior branch of the right portal vein was displaced by the mass forming its anterior margin. The posterior branch of the right portal vein was not seen. The hepatic artery was not hypertrophied. The rest of abdomen was unremarkable. On MRI (figs 6-11), the lesion was hyperintense on T2W image and hypointense on T1W image – conforming its cystic nature. Multiple septa were best seen on T2 W images which demonstrated the complex nature of the cystic mass.
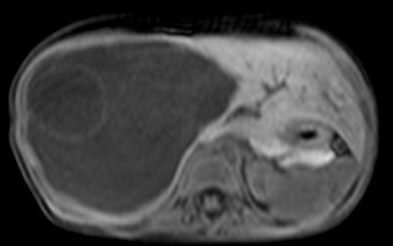
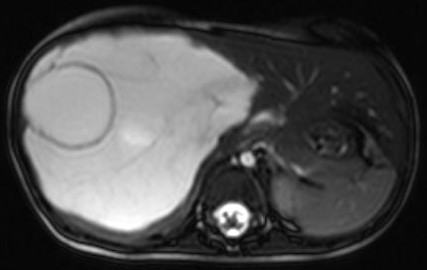
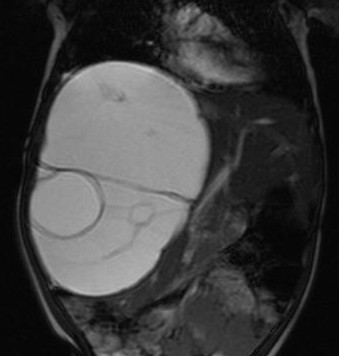
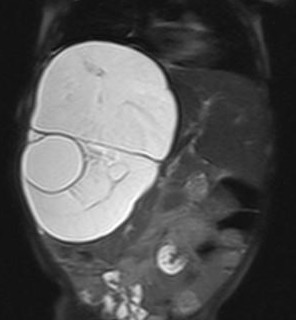
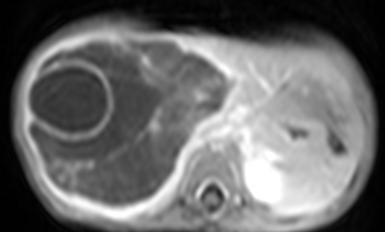
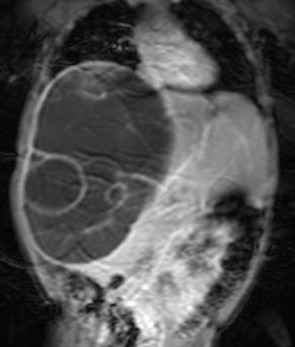
These features suggested a diagnosis of benign mesenchymal hamartoma of the liver.
Discussion:
Incidence:
Mesenchymal hamartomas are uncommon and account for only 8% of all childhood liver masses.
This lesion, though uncommon, is the second most common benign liver tumor of the pediatric
age.
Age and presentation:
It occurs exclusively during infancy and childhood although few cases in older age groups have been reported. A majority of the cases present at a mean age of 16 months, the range being from newborn to five years of age. The origin is mostly from the right lobe of the liver. There is slight male predominance. Most of the cases remain asymptomatic while others are detected incidentally. The most common clinical presentation is with a right upper quadrant mass, respiratory distress, fever and raised right hemidiaphragm. Occasionally, sudden enlargement may result from rapid fluid accumulation in the cysts. Mass effect from a bulky tumour may cause respiratory distress and lower extremity edema due to compression of the IVC. Benign liver tumors in children may be divided into two major groups:- those of epithelial derivation, including simple cysts, focal nodular hyperplasia and adenomas and those of mesenchymal derivation including hamartomas and hemangiomas. Benign mesenchymal
tumors of the liver are more common than their epithelial counterparts. A mesenchymal hamartoma is a benign cystic developmental lesion consisting of gelatinous mesenchymal tissue with cyst formation and remnants of normal hepatic parenchyma. It is a large tumor, usually of size 15cm or more at diagnosis, with cysts present in 80% of cases. It is a well-defined tumor that may be encapsulated or pedunculated. On cut section, this tumor may either have mesenchymal predominance (a solid appearance) or cystic predominance (multiloculated cystic masses).
Microscopically, it consists of cysts, remnants of portal traids, hepatocytes, and fluid filled mesenchyme. Liver function tests usually remain within normal limits. Tumor markers are negative. On CT, mesenchymal hamartomas appear as well-defined masses with central area of low density and internal septations. Both solid and cystic areas are seen. The MR appearances of mesenchymal hamartomas depend on their mesenchymal (stromal) or cystic predominance. Lesions with mesenchymal predominance have lower intensity than normal liver on T2W images because of the fibrotic tissue content. Cystic predominant lesions are of variable intensity on T1W images and are significantly hyperintense on T2W images because of the cystic component. Multiple septae are best seen on T2W images, which demonstrates the complex nature of the cystic mass. Extensive surgery is not necessary because mesenchymal hamartomas are not “true” neoplasms, but a failure of normal development – thus, simple excision, marsupialization, or incisional drainage is all that is required.
Differential diagnosis:
Vascular lesions of mesenchymal origin are hemangioendothelioma and cavernous hemangioma. Hemangioendothelioma usually presents before six months of age and is more common in females. It may be asymptomatic or presents as hepatomegaly, abdominal mass or high output cardiac failure. On CT scan, hypodense well defined homogenous masses are seen with calcification being seen in 40% of cases. Early peripheral enhancement with gradual central filling is evident. Cavernous hemangioma occurs in all age groups and is often asymptomatic and discovered incidentally. It is usually a solitary lesion with predilection for the right lobe and female population. On CT scan, it is seen as a hypodense to isodense well defined mass. On contrast enhancement, early dense, peripheral nodular enhancement with later centripetal fill is noted. In contrast, simple non-parasitic cyst is solitary which may be very large with lining of columnar, cuboidal or flattened epithelium. The wall is thin and composed of mature connective tissue. Simple cysts may also show calcification. Polycystic liver disease on the other hand is frequently associated with polycystic kidneys in about 50% of the patients. Frequently, there is cystic disease of other organs including spleen, pancreas, ovaries and lungs. The cysts may be scattered diffusely or restricted to one lobe – usually the left. Hepatic function is excellent as liver cells are preserved. CT scan is useful in diagnosing the lesion as it does not show enhancement with intravenous contrast. It may remain
asymptomatic – although portal hypertension is common in the infantile variety. Symptomatic
patients are usually in the 4th or 5th decade and symptoms are often due to associated polycystic
kidneys.
September 2007
Case contributed by Dr. Chetna Wasnik
Case History:-
A 23-year-old man was admitted with dyspnea on exertion (NYHA grade 3) since one month. There was history of palpitations on and off. Physical examination and laboratory tests were within normal limits.
Investigations:-
A 2D echocardiogram was suggestive of a sinus of Valsalva aneurysm (Figs1A and1B).
1A, 1B
The sinus of Valsalva aneurysm appeared to be partially thrombosed and unruptered and appeared to arise from the left aortic cusp.
Plain and contrast enhanced Cardiac MRI was performed.
Fig. 2A. Haste; axial image (black blood) shows the lumen of the aneurysm as also the thrombosed part.
2A
Fig. 2B TruFisp coronal image (white blood) shows the aneurysm to be arising above the left aortic cusp.
2B
Figs. 3A and 3B Post contrast images axial and coronal.
Patient then underwent a preoperative coronary angiogram.
Figs. 4A and 4B show opacification of the aneurysm and its communication.
4A and 4B
DISCUSSION:-
Thurman first described a Sinus of Valsalva aneurysm (SVA) in 1840. SVA is a rare congenital anomaly accounting for 0.5-3% of all congenital cardiac anomalies.
SVA is usually clinically silent but sometimes may present with compression of the adjacent structures or intracardiac shunting caused by rupture of the aneurysm. Approximately 65-85% of SVAs arises from the right sinus of Valsalva followed by the non coronary (10-30%) and left coronary (less than 5%) cusps.
Anatomy
:- :- Sinuses of Valsalva are spaces bounded medially by the aortic valve cusps and laterally by the wall of the aorta. There are three sinuses – right, left and noncoronary. The right coronary sinus is adjacent to the pulmonary tract, the crista ventricularis of right ventricle and the anterior portion of the right ventricle. The left coronary sinus is adjacent to the anterior wall of the left ventricle. The non coronary sinus is adjacent to the right atrium near the atrio-ventricular node and bundle of His.
Age
:- Unruptured SVAs are detected insidiously on 2D ECHO even in patients older than 60 years of age. Most ruptured aneurysms are seen in the young individuals in the second or third decades.
Sex:–
Males are affected more than the female. (M: F-4:1).
Presentation:
Approximately one quarter of patients with SVAs are asymptomatic.
Dyspnea is the most common presenting symptoms.
Rupture of the aneurysm may occur spontaneously or be precipitated by trauma, exertion or cardiac catheterization. Ruptured SVAs present with specific signs of left to right shunting and they are indistinguishable from coronary arterio-venous fistulas. Those signs are machine type continuous murmurs, bounding pulse, palpable thrill along right or left sternal border.
Ruptured SVAs progress in three stages described by Blackshear.
1) Right chest or right upper quadrant pain
2) Sub acute dyspnea on exertion or at rest
3) Progressive dyspnea, cough, edema and oliguria.
Etiology:–
Primary – Congenital (Idiopathic).
Secondary-
Atherosclerosis
Syphilis.
Cystic medial necrosis; Marfan syndrome.
Blunt or penetrating chest injuries.
Infective endocarditis.
Sinus of Valsalva aneurysms are associated with ventricular septal defect, aortic insufficiency and coarctation of aorta.
Pathophysiology:-
The aneurysm is thought to arise from incomplete fusion of the distal bulbar septum that divides the aorta and the pulmonary artery. It attaches to the annulus fibrous of the aortic valve. It is postulated that after exposure to long standing high pressures, this is the part that forms the sinuses of Valsalva -weakens and becomes aneuysmal.
Imaging:-
3) 2D ECHO may detect as many as 75% of the SVAs. It may show
– the origin of the sinus
– extension of the sinus.
– associated cardiac conditions.
4) Cardiac MRI-Multiplanar imaging combined with contrast images helps in better delineation of the site and extent of the aneurysm for preoperative assessment.
Treatment:-
Medical management-It usually involves stabilization and preoperative assessment of the patient.
Transcatheter closer of the sinus of Valsalva is done by using the Amplatzer device.
Surgical treatment-It is mainly for a patient with a ruptured aneurysm.
The surgical procedure includes
– Aortic root reconstructions or replacement.
– Aortic valve replacement of repair.
– Bentall procedure (valve conduit)
– VSD or ASD repair.
– Primary suture closures and patch closures.
Complications:-
1) Myocardial infarction due to coronary artery compression from the aneurysm.
2) Complete heart block due to compression of the conduction tissue by the aneurysm.
3) Right ventricular outflow obstruction.
4) Sudden cardiac death.
5) Infective endocarditis.
6) Tamponade if rupture occurs into the pericardium.
Outcome:-
The prognosis depends upon size of aneurysm and whether it is ruptured or not. Unruptured SVAs need to be followed up to monitor increase in size.
Most of the SVAs increase in the size and rupture. Patients with ruptured SVAs die of heart failure or endocarditis within one year of the onset of symptoms.
August 2007
Case contributed by Dr. Suvarna Barhate
Case Presentation
A three-year-old child presented with history of recurrent cough and cold since two years and failure to gain weight. Over a period of the past few months, she had often been investigated for recurrent lower respiratory tract infection and failure to thrive. She had been unsuccessfully treated with antibiotics.
Chest radiograph on admission as well as those acquired at various occasions prior to this admission, revealed increased transradiancy of the left lung field with the mediastinum being shifted to the right.(Figs 1 & 2).
The differential diagnosis of a congenital lobar emphysema and obstructive lesion of the left bronchus was considered and, for further evaluation, a CT scan of the chest was performed. This revealed an anomalous pulmonary vein coursing through the right lung and draining into the infradiaphragmatic vena cava (Figs 3-8).
An abnormal branching pattern of the right main bronchus was noticed. An anomalous “upper lobe” bronchus was observed arising form the right main bronchus – being associated with a unilobar right lung. The right main pulmonary artery appeared to be hypoplastic and monosegmental l(Figs 9-12)
The anomalous pulmonary vein draining into the infradiaphragmatic IVC can be better appreciated on the sagittal reconstructed image (Fig 13)
A diagnosis pf Scimitar syndrome was made.
Discussion:
Scimitar syndrome is a rare pulmonary anomaly with abnormal pulmonary venous drainage below the level of diaphragm. It represents a constellation of anomalies that could present variably in any particular case.
The anomalies seen in Scimitar syndrome are:
1. Anomalous pulmonary venous drainage in the IVC/Hepatic vein/Portal vein
2. Right lung hypoplasia
3. Small right pulmonary artery
4. Dextroposition of the heart
5. Anomalous systemic arterial supply to part of right lung from the aorta.
6. Cardiovascular anomalies
a. ASD
b. VSD
c. Coarctation of Aorta
d. Abnormalities of the aortic arch
e. Anomalous relationship of the pulmonary arteries and bronchi
7. Diaphragmatic anomalies
a. Accessory hemidiaphragm
b. Hepatic herniation
c. Phrenic cyst
d. Sequesteration
Various synonyms that have been used for Scimitar syndrome are as follows
1. Congenital Pulmonary venolobar syndrome
2. Hypogenetic lung syndrome
3. Epibronchial right pulmonary artery syndrome
4. Mirror image lung syndrome
The syndrome represents a constellation of above mentioned features – though it is very rare to find all of them in a given patient. Anomalous pulmonary vein and right lung hypoplasia are the most consistent findings.
The word & quot;scimitar" is probably derived from French cimeterre or directly from Italian scimitarra and is appplied to any sword with a curved blade (Fig 14)..
The silhoutte caused by the anomalous pulmonary vein draining into the infrahepatic IVC resembles a Scimitar (sword with a curved blade) , and hence the name. The respiratory system develops from the ventral wall of the foregut at 3–4 weeks’ gestation. The tracheo-bronchial tree develops between days 24 and 36 of gestation. At
28–30 days, the lung buds continue to elongate, forming the primary bronchi. The right lung grows faster than the left, being both larger and having more generations of bronchial branching. The pulmonary artery develops from the sixth aortic arch and gives off branches that parallel the development of the airways. The common pulmonary vein
becomes incorporated into the many pulmonary veins that drain into the left atrium. As the development and growth of the tracheo-bronchial tree and the pulmonary venous system are parallel, anomalies in the development of one system would adversely affect the other system and would hence, coexist. Clinical presentations of this syndrome are variable ranging from asymptomatic adults to respiratory distress in a child. Severe pulmonary hypertension and subsequent cardiac failure is a commoner presentation in children than in adults, in whom it is often an incidental finding. A classic triad that should alert a physician regarding the possibility of Scimitar syndrome includes respiratory distress, right lung hypoplasia and dextroposition of the heart. Chest radiograph would reveal dextrocardia , scimitar vein and non specific pulmonary disease in the right mid and lower zones. Often, this is because of pulmonary venous congestion secondary to inadequate drainage via the scimitar vein. Clinical suspicion is usually that of infection that fails to clear in spite of adequate antibiotic treatment. This is when, usually a CT scan of the chest is called in for. CT Chest reveals abnormal pulmonary venous drainage into the infradiaphragmatic IVC/hepatic vein, anomalies of the tracheobronchial tree, and right lung hypoplasia or unilobar right lung and anomalies of the aortic arch. 2D ECHO is indicated to detect the presence of intra and extra cardiac shunts and to quantify the magnitude and directionality of the shunt. Cine MR with 3D Contrast Enhanced MR angiography are fast becoming the one stop shop in the evaluation of patients in whom the Scimitar syndrome is suspected. Cardiac MRI accurately delineates the anatomical details of the pulmonary arterial as well as venous system, the exact anatomy of the tracheobronchial tree and presence of sequesteration. An added advantage is that of accurate quantification of intracardiac and extra cardiac shunts as well as the presence of pulmonary hypertension that has an important bearing on the further management of the patient.
3D MR angiography may obviate the need for invasive cardiac catheterization in these patients with accurate delineation of the shunts and anomalous vessel anatomy.
There are two indications for surgical intervention:
1. Large left/right shunts exceeding 50%, resulting in pulmonary hypertension and heart failure
2. Lung sequestration and/or recurrent right-sided chest infections Surgical treatment is best managed in two steps:
1. Ligation of the anomalous systemic arteries and reimplantation of the scimitar vein into the left atrium
2. Resection of sequestered or chronically infected lung parenchyma Surgical intervention should be limited to those patients with lung sequestration or recurrent serious chest infections of the affected lung or those with right
ventricle overload due to a major left/right shunt. As the syndrome is rare and less number of patients undergo surgical treatment, knowledge regarding the efficacy and complications of the procedure is limited.
Thrombosis or fibrosis of the redirected scimitar vein is a serious complication of the surgical reimplantation procedure, often needing rethoracotomy with resection of the remaining lung.
Prenatal diagnosis of Scimitar syndrome is suspected on fetal 2D ECHO on finding pulmonary venous confluence behind the right atrium and presence of a vertical pulmonary vein.
May 2007
Case contributed by Dr. PRASHANT NAPHADE
CLINICAL PROFILE:
Case Report: A 25-year-old woman G7P2A4L1 with two previous Cesarean sections presented with a history of amenorrhea of three months’ duration with mild, dull-aching pain in the lower abdomen of one week’s duration and per-vaginal blood-stained sticky discharge for the past few days. On examination, vitals were stable. Per-vaginal examination revealed an uterus of eight weeks size and a closed cervical os.
INVESTIGATIONS: The urine pregnancy test was positive. The beta HCG level was 3000 IU/L The Hb: was10 gm %
RADIOLOGICAL FINDINGS:
The patient was admitted and a USG was performed. This showed a mixed echogenic lesion with a central cystic area in the lower uterine segment separate from the endometrial canal displacing the endometrium posteriorly. On Doppler examination, pericystic vascularity was detected. The ovaries were normal bilaterally . No free fluid was detected in the pelvis.
FIG 1,2
MRI:
T2WI revealed a 6.8 x 6.7 x 6.1 cm, well circumscribed lesion in the myometrium of the lower uterine segment which showed marked T2 shortening along the periphery with a central cystic area showing hyperintense signal. The lesion was separate from the urinary bladder and endometrial canal. Healthy myometrium was not seen between the mass and the bladder.
Fig 3 ,4
Fig 5, 6
In view of the history of previous Cesarean section and the location of the gestational sac and elevated beta HCG levels above the discriminatory cut off value of 1,500-2,500IU/L, a final diagnosis of ectopic gestation in a Cesarean section scar was made
The patient was treated with IV Methotrexate 66mg% (50 mg/sq.m). Follow up beta HCG levels had dropped to 1700 at one week and 400 at 15 days. The patient was discharged. Currently she is on follow up.
DISCUSSION:
C<
esarean scar pregnancy is the rarest form of ectopic pregnancy. Less than a 100 cases have been reported so far.
A uterine scar pregnancy is a gestation separate from the endometrial cavity and completely surrounded by the myometrium and the fibrous tissue of the scar. The most probable mechanism for its occurrence is the invasion of the myometrium by a microscopic tract. In 60% to 70% of Cesarean scar pregnancies, there is clear evidence of the trophoblast penetrating the endometrial-myometrial junction. It has been postulated that first trimester cesarean scar pregnancies that invade the myometrium may develop into placenta previa/accreta if the pregnancy is allowed to progress.
ETIOLOGY:
The tract is believed to develop from trauma from previous uterine surgeries like dilatation and curettage, myomectomy, metroplasty and caesarean section.
CLINICAL FEATURES:
Amenorrhoea of 2 to 3 months followed by vaginal bleed. History of previous operative procedure as mentioned above.
COMPLICATIONS:
Hemorrhage and uterine rupture are dreaded complications often occurring in the first trimester.
USG:
Transvaginal ultrasonography (TVUS) combined with Doppler is a reliable tool for the diagnosis. TVUS, is the most useful imaging tool in the diagnosis of ectopic pregnancy. It is non-invasive, readily available and accurate.
Ultrasound imaging criteria to diagnose Cesarean scar pregnancy are as follows:
1) Empty uterine cavity and cervical canal;
2) Development of the gestational sac in the anterior uterine wall at the isthmus (presumed site of the previous lower segment caesarean section scar);
3) Evidence of functional trophoblastic circulation on Doppler examination, defined by the presence of an area of increased peritrophoblastic vascularity on colour Doppler examination
4) The absence of healthy myometrium between the bladder and sac, allowing differentiation from cervico-isthmic implantation.
This entity is to be should be distinguished from two conditions, cervical pregnancy and miscarriage-in-progress.
In cervical pregnancy, the endocervical canal can be seen bulging with gestational products
In miscarriage-in-progress, there is no functional peri-trophoblastic circulation on Doppler examination, and probe pressure on the cervix will show that the sac can be displaced.
The combination of non diagnostic sonographic findings and a serum HCG level above the discriminatory zone are highly specific for a diagnosis of ectopic pregnancy .When the serum human chorionic gonadotrophin level is above the discriminatory cutoff value of 1,500-2,500IU/L, a normal intrauterine pregnancy should always be detected by TVUS
MRI
An irregularly-marginated mass with very heterogeneous signal intensity on T2-weighted images, irregular internal high-signal intensities on T1-weighted images, a partial or circumferential rim of low-signal intensity, dense irregular peripheral enhancement and enhancing papillary solid components with accompanying tubular signal voids, and variably increased parametrial vascularities. This heterogeneous hemorrhagic mass with densely enhancing solid papillary components may be the typical MR finding .
TREATMENT:
Most other case reports involving conservative management have used methotrexate either systemically or by direct injection into the pregnancy sac or a combination,
There are also different regimens of medical treatment—single and multiple dosage. Due to the rarity of scar pregnancy, it is impossible to conclude whether systemic or local methotrexate administration is safer or more effective. Local administration of methotrexate avoids the systemic side-effects and maybe more effective if the initial HCG level is higher than
10 000 IU/L. Systemic administration of methotrexate may be used for an early scar pregnancy with an HCG of <10 000 IU/L. In all other cases, and those where expectant or systemic methotrexate treatment fails, the choice should be surgery or ultrasound-guided local medical treatment with or without UAE, depending on local expertise and practice and experience. Surgical treatment is necessary for clinically unstable patients and where there is large amount of fluid in the pelvic cavity on the ultrasound scan.
Beta HCG usually takes 6 to 10 weeks to reach undetectable level, but it may take upto six months.
April 2007
Case Contributed by Dr. Yogesh Yadav
Clinical Profile
A 22-year-old male presented with the complaint of pain on the left side of chest on deep inspiration for two months and dyspnea on exertion for one month. There was no history of breathlessness or trauma to the chest. On examination, vital parameters were normal. Cardiovascular examination revealed a loud first heart sound and audible fourth heart sound; the second heart sound was normal.
RADIOLOGICAL FINDINGS
Frontal and lateral chest radiographs (Fig 1, 2) showed an approximately 5 cm x 4 cm well defined soft tissue density mass lesion in the region of left hilum, obscuring the left heart border. The lesion showed a discontinuous rim of
calcification. The left hilum was seen through the lesion suggesting that it was separate from the left hilum. The lung fields were normal.
Fig 1,2
Plain and contrast enhanced CT scan of the chest showed an approximately 6 cm x 5 cm x 5 cm sized peripherally calcified lesion in relation to the anterosuperior wall of the left ventricle. There was intense enhancement on post
contrast scan. (Figs 3,4,5)
Figs 3, 4,5
Cardiac MRI was done next. This revealed an approximately 6 cm x 5 cm x 5 cm sized pseudoaneurysm arising from the anterosuperior wall of the left ventricle. This sac filled during systole and a jet was seen extending within the aneurysm. The lesion was in close proximity to the mitral valve but seen separate from it. The left main pulmonary artery and left main bronchus were displaced superiorly due its mass effect. Post contrast dynamic angiography shows no clot or thrombus within ventricles. (Figs 6-12)
Figs 6-13
2D ewchocardiography also reveled similar findings.
A diagnosis of a left ventricular pseudoaneurysm was made.
DISCUSSION:
Left ventricular aneurysms are large thin walled fibrous sacs bulging from the lumen of the left ventricular cavity and also from the external surface of the heart and are usually clearly demarcated from the normal myocardium. A
pseudoaneurysm is a rupture of the myocardium that is contained by pericardial adhesions.
AETIOLOGY:
Over 95% of true left ventricular aneurysms result from coronary artery disease and myocardial infarction. True left ventricular aneurysms also may result from trauma, Chagas' disease or sarcoidosis. A very small number of congenital left ventricular aneurysms also have been reported and have been termed
diverticula of the left ventricle. False aneurysms of the left ventricle result most commonly from contained
rupture of the ventricle 5 to 10 days after myocardial infarction and often occur after left circumflex coronary arterial occlusion. False aneurysms of the left ventricle also may result from submitral rupture of the ventricular wall after mitral valve replacement. Left ventricular pseudoaneurysm may also result from septic pericarditis or any prior operation on the left ventricle, aortic or mitral annulus.
Location:
One of the most easily documented features proposed for distinguishing true aneurysms from pseudoaneurysms is location. Plain chest radiography often reveals pseudoaneurysm, particularly when there is a discrete bulge in the
cardiac shadow in an atypical location for ordinary cardiomegaly, such as posteriorly. It has been suggested that an inferior or posterior location is suggestive of pseudoaneurysm rather than a true aneurysm.
Symptoms and Examination Findings:
Angina is the most frequent symptom in most series of patients operated upon for left ventricular aneurysm. Dyspnea is the second most common symptom of ventricular aneurysm and often develops when 20% or more of the ventricular wall is infarcted. Either atrial or ventricular arrhythmias may produce palpitations,
syncope or sudden death or aggravate angina and dyspnea in up to one third of patients.
Symptoms of pseudoaneurysm include recurrent chest pain which may be associated with symptoms of hypotension. Signs of a pseudoaneurysm include decreased heart sounds, a pericardial friction rub, elevation of both left- and right-sided filling pressures, and sinus bradycardia or junctional rhythm. When the pseudoaneurysm is large, and it may produce an apical impulse. Mechanical interference of the mass on ventricular filling may result in a third heart sound. A pansystolic or to-and-fro murmur may be produced by flow across the mouth of the pseudoaneurysm. The ECG often shows persistent ST segment elevation in the area of the infarct. Unfortunately, all of these signs and symptoms are also characteristic of true aneurysms.
Imaging Characteristics:
Contrast Ventriculography
Left ventriculography is the gold standard for diagnosis of left ventricular
aneurysm. The diagnosis is made by demonstrating a large, discrete area of
dyskinesia (or akinesia), generally in the anteroseptal-apical walls. Occasionally,
left ventriculography also may demonstrate a mural thrombus. Outward motion is
termed dyskinetic, and the remaining aneurysmal segments are termed akinetic.
The characteristic feature of pseudoaneurysms is a narrow neck connecting the
ventricle to the pseudoaneurysm cavity. Radionuclide ventriculography may be used for the diagnosis.
Magnetic Resonance Imaging:
The advantages of MRI are its high spatial resolution and ability to image the entire heart. Thus, it is highly accurate in determining the size and location of the pseudoaneurysm. It is also capable of distinguishing between pericardium, thrombus, and myocardium and the potential to visualize disruption of the epicardial fat layer by the pseudoaneurysm.
Echocardiography:
Two-dimensional echocardiography is also a sensitive and specific means of diagnosing left ventricular aneurysm .Mural thrombus and mitral valve regurgitation are detected most readily by echocardiography. Echocardiography
is also useful for distinguishing pseudoaneurysm from true aneurysm by demonstrating a defect in the true ventricular wall. Trans Esophageal Echocardiography (TEE) is the modality most studied with
respect to distinguishing ventricular aneurysms from pseudoaneurysms. The nature of flow within a pseudoaneurysm has been used to distinguish it from true aneurysm based on results with echocardiographic Doppler techniques.
The presence of turbulent flow by pulsed Doppler at the neck of a cavity or within the cavity itself suggests pseudoaneurysm.
Natural History:
Frank rupture of chronic left ventricular pseudoaneurysms is less common than one might expect. Rupture of left ventricular pseudoaneurysms may be most likely in the acute phase or in large-sized pseudoaneurysms. Left ventricular pseudoaneurysms tend to behave similar to true aneurysms in that they may present a volume load on the left ventricle or may be a source of embolization or endocarditis. Left ventricular pseudoaneurysms after prior cardiac surgery have also been reported to compress adjacent structures such as the pulmonary artery
or esophagus.
Treatment:
Surgery is the treatment of choice for pseudoaneurysms due their propensity to rupture while true aneurysms can be managed medically in low risk patients.
Case of the month March 2007
Case contributed by Dr. Prabath Mondel
A 35-year-old man presented with sudden onset of acute abdominal pain in the right upper quadrant. He had been having progressively increasing yellowish discoloration of sclera, pale stools and generalized pruritis since several weeks. On examination, jaundice and mildly tender, mild hepatomegaly were the only positive findings. The vital signs were normal.
INVESTIGATIONS: Hb : 11.3 gm% ,TLC: 12800/cmm; Sr. bilirubin .: 25.9 mg %,
The patient was admitted and a CT scan was performed. This showed a cyst in segment 4 A & b with obstructive biliary dilatation. Reconstructed images clearly showed the cystic lesion in liver in communication with common the bile duct.
Fig 1, 2
An MRCP showed an approximately 7 cm diameter hypointense lesion in segments 4 a & b with linear hyperintense band within causing obstructive intrahepatic biliary dilatation .
The T2 weighted HASTE sequence showed a cystic lesion with septations within associated with obstructive biliary dilatation. The coronal images demonstrated communication of the cyst with the common bile duct.
Fig 3, 4, 5
Post contrast MR: showed peripheral enhancement of the cyst in segments 4 a & b with hypertrophy of the left lobe of the liver. The gall bladder was distended .The suprapancreatic common bile was dilated with gradual tapering of intrahepatic common bile duct.
Fig ,6,7
A percutaneous trans-hepatic biliary drainage was performed.
However, the patient developed a peri-catheter leak and was operated with excavation of the cyst. A T tube was placed to drain the bile. The patient recovered well following the procedure and a T tube cholangiogram was performed. This showed a small residual cavity filled with contrast and no leak.
Discussion:
Hepatic hydatid disease (HHD) is a major endemic problem in sheep-rearing regions of the world. Man is the incidental host affected accidentally when he comes into contact with food contaminated with dog feces or when he comes in close contact with sheep. The liver acts as a filter for hydatid larvae, making it the most commonly affected organ. Up to one-third of patients with HHD present with complications such as rupture (into the biliary tree, thorax or peritoneum), secondary infection, anaphylactic shock and sepsis.
HHD disease is the commonest form of echinococcosis. The right lobe of the liver is affected in 80% of cases and the left lobe in 20% of cases. Rupture occurs into the right duct in 60% of cases, into the left duct in 30%. Intrabiliary rupture can lead to obstructive septic or allergic manifestations. Patients commonly present with right upper abdominal pain (82%), obstructive jaundice (57–100%), fever (70–90%), acute cholangitis (20–37%), abdominal lump (22–39%), and rarely with acute pancreatitis, liver abscess or septicaemia, or it may be asymptomatic (5–6%). Lewall and McCorkell have classified rupture of echinococcal cysts into three types: contained, communicating and direct.
Ultrasound is the most commonly employed initial investigation.
A complicated cyst has a multivesicular / multiseptate appearance
with a heterogeneous echogenic interior. A dilated CBD in
a jaundiced patient with a hydatid-like cystic lesion in the
liver should prompt a diagnosis of intrabiliary rupture. Extrahepatic biliary dilatation
is a constant feature. Echogenic or non-echogenic material without
posterior acoustic shadowing is seen in the biliary tree, suggestive <
of sludge and daughter cysts.
The features of a hydatid cyst on CT are enhancement of the
cyst wall and the internal septe; visualization of detached undulating membranes and calcification of the cyst wall. A dilated CBD with low attenuation intraluminal material
suggests the presence of hydatid sand and cysts in the CBD.
An interrupted area of the cyst wall proximal to a dilated duct
may be identified as representing the site of communication.
Cyst wall discontinuity – a direct sign of rupture, was seen
in only 75% of cases. CT can demonstrates high attenuation
material passing through the defect of the cystic wall and filling
up the intrahepatic biliary radicles or CBD.
MRI is a useful tool in difficult cases such as intrabiliary rupture, where CT and ultrasound are not conclusive. The wall of the hydatid cyst is seen as a low intensity rim, a reliable sign to differentiate hydatid cyst from other simple cysts. Daughter cysts have a lower signal intensity compared with the mother cyst. The MRI finding in ruptured hydatid cyst can be direct or indirect. A breach in the low intensity rim of the cyst wall with extrusion of cyst contents is a direct sign, while increased echogenicity, fluid levels, presence of air and changes in signal intensity are indirect signs.
ERCP is the gold standard in confirming biliary tract involvement and may be of therapeutic benefit in selected cases. On ERCP, a swollen ampula of Vater may be seen, with hydatid material protruding out. Dilated ducts with debris and daughter cysts may appear as radiolucent filling defects. Irregular leaf-like material that changes shape with changes in pressure differentiates this condition from other causes of obstructive jaundice. A small cysto-biliary communication cannot always be excluded by ERCP and should be actively sought during surgery.
The usual findings in HIDA scan are photopenic areas in the liver in initial images, which gradually fills up in delayed images indicating bile leak into the cyst. Although it cannot document the exact nature of communication, HIDA can be helpful in doubtful cases with cysto-biliary communication where ultrasound and CT are not conclusive.
Surgery is considered the ideal treatment though percutaneous sclerotherapy has been used with varying success rates.
Case of the Month December 2006
Case contributed by Dr. Abhishek Keraliya
CLINICAL PROFILE
A 62-year-old man presented with pain in the abdomen radiating to the back and dyspepsia since one month. There was no history of any other major illness. On examination, there was a well defined palpable mass in the epigastrium associated with mild tenderness. Routine blood investigations were normal. Serum amylase was normal.
Serum lipase level was increased (1531 IU/L). Serum levels of CA 19-9 (tumor marker for pancreatic carcinoma) were normal.
RADIOLOGICAL FINDINGS
Ultrasonographic findings: There was a large, well-defined, hypoechoic mass (approximately 12x10x10 cm in size ) arising from the body of the pancreas.
CT findings (Figs 1-5):
A soft tissue density mass was noted in the upper abdomen – arising from the body of the pancreas and extending anteriorly and cranially- causing encasement of the celiac artery and its branches and occupying most of the lesser sac. The mass showed minimal homogenous enhancement after intravenous contrast administration. There was loss of fat planes between the mass and the stomach and the left lobe of the liver. There was no calcification seen in the mass. Incidentally noted were a right renal cyst and a simple cyst in the right lobe of the liver. A few calcified
granulomas were also seen in spleen.
Figs. 1-5
MR findings: (Figs 6-9) On T1W images, a well defined homogenously hypointense mass was seen in the upper abdomen, arising from the body of the pancreas. The lesion appeared hyperintense on T2W images .The mass was seen invading the anterior structures including the posterior wall of stomach and the left lobe of the liver. The
pancreatic duct was not dilated. The tail of the pancreas was normal. The mass showed encasement of vessels including the celiac artery and its branches and the splenic vein. The liver and spleen were of normal size.
USG guided biopsy:
An ultrasound guided Tru-Cut biopsy was performed under local anesthesia. .Histopathological evaluation revealed sheets of small round cells of apparent lymphoid origin. The cells showed hyperchromatic nuclei and scanty cytoplasm. These features were suggestive of low grade malignant lymphoma (NHL).
Final diagnosis: Pancreatic lymphoma
DISCUSSION
In lymphoma, primary involvement of the pancreas is uncommon, representing 2% to 5% of cases of extranodal lymphomas, comprising less than 0.5% of pancreatic tumours. Many of these cases occur in patients who are immunocompromised hosts – particularly
patients infected with HIV.
Lymphoma, predominantly the non Hodgkin B cell subtype, involves the pancreas
secondarily in approximately 30% of patients with widespread disease. It usually spreads
to the pancreas by direct extension from peripancreatic lymphadenopathy.
To distinguish PPL (primary pancreatic lymphoma) from secondary involvement of the
pancreas by non-Hodgkin's lymphoma, Behrns' clinical and diagnostic criteria of PPL
include:
1. Mass predominantly within the pancreas with grossly involved lymph nodes confined
to the peripancreatic region
2. No palpable superficial lymphadenopathy
3. No hepatic or Splenic involvement
4. No mediastinal nodal enlargement on chest radiograph
5. Normal white cell count.
AGE: The disease mainly affects middle age and elderly patients
SEX: Male to female ratio is around 1.4: 1
PRESENTING SYMPTOMS are non-specific, typically including abdominal pain,
weight loss, nausea and vomiting, but also jaundice, acute pancreatitis, and small bowel
obstruction.
RADIOLOGICAL FINDINGS:
USG Findings:
Sonography reveals a homogeneous, sonolucent, or complex mass. These masses are usually echo-poor and may mimic cystic lesions. Transabdominal sonography allows the detection of enlarged peripancreatic and periaortic lymph nodes and dilatation of the common bile and pancreatic ducts. Doppler waveform scanning, provide information about the patency of the major peripancreatic vessels, the celiac and superior mesenteric arteries, and the portal, superior mesenteric, and splenic veins.
CT Findings:
CT is the most common imaging technique used in the detection and characterization of primary pancreatic lymphoma.
Pancreatic lymphomas generally appear as homogeneous soft tissue mass, showing little enhancement after intravenous contrast administration. Intrinsic involvement of the pancreas may be difficult to differentiate from lymphoma affecting the peripancreatic
lymph nodes.
Two distinct CT patterns have been described, including focal and circumscribed single
or multiple masses and diffuse enlargement of the gland by an infiltrating tumor. The
latter appearance can be associated with involvement of the peripancreatic fat and mimic
acute pancreatitis on CT. Encasement of the peripancreatic vessels may occur, but dilatation of the pancreatic duct
is uncommon, despite the presence of bulky tumor, a helpful distinguishing feature from
adenocarcinoma. The presence of associated lymphadenopathy below the level of the renal veins also
favors the diagnosis of lymphoma.
MR Imaging :
Two different morphologic patterns of pancreatic involvement are seen on MR imaging
that are similar to the CT appearance. The well-circumscribed tumoral type appears as a low-signal-intensity homogeneous mass within the pancreas on T1-weighted images with subtle enhancement after IV administration of gadolinium-containing contrast medium. On T2-weighted images, a tumoral mass shows a more heterogeneous character with a low to intermediate signal amplitude slightly higher than that of the residual gland but much lower than the signal intensity of fluid.
The diffuse infiltrating type of pancreatic involvement shows similar characteristics of low signal intensity on unenhanced T1- and T2-weighted images, with mild to moderate enhancement after gadolinium injection. In the diffuse infiltrating type, enhancement is predominately homogeneous but may include small foci of little or no gadolinium uptake. Bile and pancreatic ductal dilatation can be easily assessed with MR imaging using MR
cholangiopancreatography .
ERCP and Percutaneous Transhepatic Choledochography
Unlike pancreatic adenocarcinoma, moderate to severe dilatation of Wirsung's duct is apparently rare in pancreatic lymphoma because Wirsung's duct is either normal, displaced or simply narrowed in patients with pancreatic lymphoma. Bile duct dilatation from obstruction is seen more often because jaundice occurs in 42%
of patients with non-Hodgkin's lymphoma primarily involving the pancreas.
Diagnosis, Treatment, and Prognosis:
As the prognosis of a pancreatic lymphoma is favorable, its differentiation from a carcinoma is crucial. The correlation of USG , CT and MR findings may result in a correct diagnosis. However, if doubt exists, biopsy may reveal the true nature of the mass.
Percutaneous(usg guided) or endoscopic core biopsy should be performed to establish the diagnosis. In most patients, the diagnosis can be established without surgery; this fact is a major reason to look for findings suggestive of pancreatic lymphoma.
Summary
When the radiologist is faced with a well-circumscribed tumoral mass in the pancreas, knowing when to direct the patient toward non-surgical biopsy instead of surgical biopsy is critical. Lymphoma does not require surgical staging or a palliative Whipple's procedure before chemotherapy or radiation therapy. In patients with primary pancreatic lymphoma, no marked pancreatic ductal dilatation is present even with ductal invasion.
Adenocarcinoma commonly dilates the more distal pancreatic duct when more proximal ductal invasion has taken place. Lymph node involvement below the level of the renal veins is another finding not seen with adenocarcinoma. Clinical and imaging findings are otherwise not specific in the differentiation of pancreatic lymphoma and pancreatic
cancer, but a bulky homogeneous tumoral mass without alteration of Wirsung's duct or the peripancreatic vessels should suggest the diagnosis. In patients with diffuse infiltration of the pancreatic gland without clinical signs of pancreatitis, the radiologist should be alert to the possibility of pancreatic lymphoma.
November 2006
Case contributed by Dr. Dipak Bankar
CLINICAL PROFILE
A 27-year-old man presented with a swelling on the right side of the neck since one year. The swelling gradually increased in size and had been associated with hoarseness of voice since seven to eight months. The patient also complained of difficulty in deglutition associated with tinnitus in the right ear. Local examination revealed a large cystic mass in the upper lateral part of the right side of the neck with mild pulsatality. Mild loss of the right naso-labial fold was noted. There was right sided mixed hearing loss. On indirect laryngoscopy, there was right vocal cord palsy.
RADIOLOGICAL FINDINGS
A CT SCAN
of the base of the skull showed a hypodense lesion (Fig.1) in the region of the carotid fossa. The lesion showed intense enhancement on post contrast images (Fig.2). There was splaying of the ECA and ICA. Another similar lesion was seen in the jugular fossa. On bone windows, there was erosion of the jugular spine (Fig.3).
Fig.1 Fig.2 Fig.3
An MRI
of the neck showed a hypointense soft tissue mass in the right jugular fossa. On T1W images, this lesion was seen to extend into the posterior fossa below the level of the external auditory canal. Multiple flow voids were seen within it. The lesion remained hypointense on T2W images as well. The lesion was in contiguity with the carotid canal anteriorly. Another soft tissue intensity mass was seen in the region of the common carotid bifurcation – hyper to isointense on T2W images. This mass produced splaying of the ECA and the ICA (Fig.4) and extended into the parapharyngeal space medially and submandibular region anteriorly. Both the lesions showed intense enhancement on post contrast scans (Fig.5&6) – showing a classic salt and pepper appearance on post contrast scans.
Fig.4 Fig.5
Fig.6 Fig.7
A
FOUR VESSEL ANGIOGRAM
was performed. This showed spaying of the carotid bifurcation (Fig.9) and an intense blush (hypervascularity) of the tumour (Fig.10). The characteristic position of the tumour gives the clue to the diagnosis.
Fig.8 Fig.9 Fig.10
FNAC of the lesion was performed. This showed it to be a paraganglioma.
Direct percutaneus glue embolisation of the right carotid body tumour and transarterial sclerotherapy of right glomus tympanicum lesion were performed. There was significant reduction in the vascularity of the lesion. This was shown by the follow up MRI angio (Fig.11) and sonography (Fig.12).
Fig.11 Fig.12
FINAL DIAGNOSIS: GLOMUS JUGULARE AND CAROTID BODY TUMOUR (MULTIPLE CHEMODECTOMAS).
DISCUSSION:
Pathophysiology:
Glomus tumors of the head and neck paraganglia are part of the extra-adrenal neuroendocrine system. At birth, small patches of paraganglionic cells can be widely dispersed throughout the body, mostly in association with autonomic nervous tissue. In the head and neck, such areas include the chemoreceptive areas (glomus tissue) of the carotid bifurcations, the aortic arch, and the temporal bone. The major paraganglia that do not undergo involution are the carotid bodies. They line the medial wall of the bifurcation of the common carotid artery. These paraganglia are functionally important chemoreceptive organ for homeostasis. Specifically, they detect changes in arterial partial pressures of oxygen and carbon dioxide and changes in pH and other blood-borne factors.
Clinical presentation:
Carotid body tumors have no sex predilection. However, studies have shown evidence of a sex predilection with jugulare tumors, with a female-to-male ratios of 5:1. Studies indicate that the incidence of carotid body tumors peaks in patients aged 45-50 years, whereas the incidence of tumors of vagal and jugular origin peaks in those aged 50-60 years. Glomus tumors of the head and neck are extremely rare in pediatric patients.
Patients with carotid body tumors are largely asymptomatic and have a mobile, nontender, slow growing, lateral neck mass. Some patients may report hoarseness and dysphagia associated with compression of the trachea and esophagus and/or vertigo and paresis resulting from cranial nerve compression. Patients with functioning tumors may present with hypertension, headaches, palpitations and tachycardia resulting from increased levels of circulating catecholamines.
Imaging studies
Contrast-enhanced CT demonstrates enhancing soft-tissue masses at characteristic locations – which is a key to the diagnosis. Nonenhanced CT imaging can demonstrate glomus tumors, but the demonstration of a strongly enhancing mass is typical. CT demonstrates carotid body tumors at the level of the carotid bifurcation, respectively splaying the internal and external carotid arteries medially and laterally. These tumors can vary in size, but their location within the bifurcation is critical for diagnosis. Similar to CT imaging, contrast-enhanced MRI demonstrates enhancing soft-tissue masses at characteristic locations. Demonstration of a strongly enhancing mass is typical in the diagnosis of a glomus tumor. As with most soft tissue tumors, glomus tumors are isointense on T1W MRI and hyperintense on T2W MRI. Contrast-enhanced imaging can show intense tumor enhancement. MRI can show densely enhancing carotid body tumors at the level of the carotid bifurcation, which respectively splay the internal and external carotid arteries medially and laterally. Sonography can demonstrate the extent of the masses and show their locations. Because of tumor neovascularity, Doppler USG sampling of cervical masses, such as carotid body tumors, can be helpful in diagnosis. Glomus tumors of the head and neck are typically highly vascular, as shown on angiograms. This finding differentiates them from other types of neck neoplasia.
Treatment
The preferred method of treatment for glomus tumors of the head and neck is surgery. However, because most paragangliomas are slow-growing and benign, radiation treatment alone or no treatment at all is preferred in elderly patients in whom the risks of surgery are relatively high and the tumor is unlikely to cause serious morbidity or mortality. If the patient is young, surgery is the best available option because it is the only option that allows total cure.
Embolization is a common technique used as the lone treatment option or as a precursor to surgical excision. As a result of the highly vascular nature of these neoplasms, embolization is an effective technique that is aimed at starving the lesion of its blood supply and inducing necrosis.
Case of the Month – October 2006
Case contributed by Dr. Chirag Khajanchi
CLINICAL PROFILE
A eight-year-old boy presented with a swelling on the posterior aspect of the head since two months associated with headache off and on. The patient had a history of fall during play two years back with resultant torticollis which subsided on its own. On local examination, there was a 8 x 6 cm, ill defined, high occipital swelling with mild pulsatility.
CNS examination was unremarkable with no focal neurological deficit. Routine hematological examinations were normal.
RADIOLOGICAL FINDINGS
A CT scan of the brain (plain) done two years back at the time of injury was normal.
FIG. 1
A CT scan of the brain at the time of the present admission revealed an expansile, permeative destructive pattern of the parietal bone involving both the inner and outer tables associated with a subgaleal soft tissue swelling. The plain scan revealed a hyperdense mass lesion involving the posterior high parietal region predominantly on the left side with multiple calcifications within. There was associated perilesional white matter edema. Post contrast CT in the early venous phase revealed multiple tortuous vessels suggestive of a vascular malformation. The proximal superior sagittal sinus revealed contrast within it. However, the distal one- third showed no contrast – suggesting thrombosis. Post contrast CT reconstructed in the coronal plane confirmed destruction of the bone with aneurysmal dilatation of the venous channels suggestive of vascular malformations.
FIG.2 FIG.3
FIG.4 FIG.5
MRI study of the brain with contrast showed a large dural AVM at the vertex. Multiple enlarged draining veins were seen. The superior sagittal sinus and the right transverse sinus were enlarged. An underlying solid-cystic soft tissue mass was noted at the vertex. This mass was predominantly on the left side and appeared to be extra axial. It caused destruction of the adjacent bony calvarium. The mass protruded into the scalp through the bony calvarial defect. The mass measured 11.4 x 10.9 x 8.9 cms in maximum craniocaudal, transverse and AP diameters. It was associated with perilesional edema and mass effect. The lesion caused compression of the left lateral ventricle, posterior corpus callosum and brainstem with mild subfalcine herniation to the right side. Subacute intralesional hemorrhage could be seen.
FIG.6 FIG.7
FIG.8 FIG.9
A four vessel angiogram showed a large, highly vascular, dural based mass on the vertex displacing the superior sagittal sinus inferiorly and extending out of the skull. This superior component of the lesion was seen to extend on both the sides of midline. There was another large component of this lesion which was seen inferior to the superior sagittal sinus in the left temporo – parieto – occipital regions. The lesion derived its supply from multiple small branches of both middle meningeal arteries, occipital arteries, dural twigs of dysplastic cortical branches of both middle cerebral arteries, anterior cerebral arteries and posterior cerebral arteries. The draining veins are hypertrophic and dysplastic with multiple ectasias and drained into the hypertrophic superior sagittal sinus.
FIG.10 FIG.11 FIG.12
The child complained of productive cough since two weeks. A chest radiograph revealed a well defined nodular soft tissue mass lesion in the right mid zone. A CT scan of the chest was performed. This showed an isodense nodular mass lesion in the right upper lobe with amorphous calcification and lobulated margins. On post contrast examination, the lesion showed heterogenous enhancement with HU of 35 – 55.
FIG.13
FIG.14 FIG.15
In view of the age of the child and pattern of bone destruction, a diagnosis of Ewings sarcoma was made. The child underwent a biopsy of the skull lesion. The histopathology and immuno histo chemistry confirmed it to be an EWINGS SARCOMA. We believe that the vascularity is related to the tumor though it is difficult to be certain that this is not a separate dural AVM secondary to the trauma.
FINAL DIAGNOSIS : EWINGS SARCOMA OF THE SKULL WITH UNDERLYING DURAL ARTERIO VENOUS FISTULAS WITH LUNG METASTASES.
DISCUSSION
INCIDENCE: Ewing sarcoma is a rare, highly malignant neoplasm of bone accounting for about 5% of biopsied bone tumours. Common sites of primary Ewing’s sarcoma are long bones (47%), pelvis (19%) and ribs (12%). Ewing’s sarcoma of skull vault is rare. It constitutes less than 1% of all the brain tumours. Frontal, parietal and occipital bones are common sites in the skull. Skull base and facial bones are less commonly involved. Seventy five percent of cases are under the age of 20 years, with peak incidence between 5 to 13 years.
PRESENTATION: Usually patients with primary skull vault Ewing’s sarcoma present with pain and swelling. Rarely, the patient may present with a neurosurgical emergency. Neurological signs and symptoms may be present when the tumour is large, compressing or invading the brain parenchyma. These signs and symptoms vary according to size of tumor and region of brain parenchyma involve
HISTOPATHOLOGY: On FNAC, Ewing’s sarcoma shows a cluster of monomorphic tumor cells with round vesicular nuclei and ill defined vacuolated cytoplasm. Many dissociated cells present with naked nuclei. Demonstration of glycogen in the cytoplasm is usually a consistent finding in Ewing’s sarcoma. Histologically, Ewing’s sarcoma is a highly anaplastic tumour with solidly packed small round cells. Light microscopic, ultrastructural and immunohistochemical features differentiate Ewing’s sarcoma from neuroblastoma, lymphoma and rhabdomyosarcoma.
SITES: The most common sites for primary Ewing’s sarcoma is long bones. Diaphysis is more commonly involved, although in 25% cases metaphyses may also be involved. Ewing’s sarcoma in long bones presents with a permeative pattern of medullary destruction a having wide zone of transition, destruction of cortex, multilamellar periosteal reaction and presence of soft tissue.
RADIOLOGICAL FINDINGS:
Primary skull lesions present with osteolytic lesion with erosion of inner and outer table associated with soft tissue swelling as seen on plain films.. Similar osteolytic lesions in the skull vault are noted in eosinophilic granuloma; Hand Schuller Christian syndrome, metastasis, Burkitt’s lymphoma, fibrous dysplasia, aneurysmal bone cysts, osteoclastoma and giant cell reparative granuloma. Radiologically it is difficult to distinguish them however, they can be distinguished by different pattern of destruction of skull vault, presence of soft tissue, calcification, cystic component and septations. However histopathology is more specific for their differentiation.
Ewings sarcoma of the skull has a tendency to create a significant epidural mass and push in to brain . The epidural mass is well seen on CT scans .The mass is usually iso to hyperdense on plain scan with heterogenous enhancement on post contrast study. MRI is useful in precise delineation of different tumour components such as extent of bone, dural and parenchymal involvement. Epidural masses rarely invade the dura and invade the brain parenchyma.
Primary Ewing’s sarcoma of the skull has lesser tendency to metastasize to lung and bones (one of few bone tumors metastasizing to bones). From remote primary sites like pelvis and long bones Ewing’s sarcoma may metastasize to skull, spine, meninges and brain parenchyma. Extensive workup is indicated in all cases of Ewing’s sarcoma of skull to search for extracranial primary sites .Radionuclide scanning is most sensitive in early detection of the primary lesion and metastatic deposits. Metastasis to brain parenchyma and dura is less frequent. CNS parenchymal involvement is more common due to primary bone tumour rather than metastatic disease.
Most primary Ewing’s sarcomas have good prognosis because they can be totally or subtotally excised. However, those arising from skull base may involve vital structures prohibiting surgical excision.
TREATMENT:
Surgical removal and /or radiotherapy with adjuvant chemotherapy are treatment of choice. Isolated radiotherapy is indicated in inoperable cases .High risk cases should receive combined radiotherapy and surgical treatment, preferably preoperative irradiation to the lesion
Patients with primary skull vault Ewing’s sarcoma have excellent prognosis with 2-5 years of disease free survival in 50-80% cases.
August 2006
Case contributed by Dr. Ramnath Ghute
CLINICAL PROFILE
A 23-year-old man presented with recurrent hemoptysis since 12 years. The hemoptysis was streaky and on and off. There is no history of tuberculosis or bleeding from other sites. Physical examination was unremarkable.Radiological findings :Frontal and lateral radiographs of the chest show a hyperlucent left hemithorax – especially in the left mid
and lower zones and a doubtful soft tissue shadow overlying the heart shadow.
Fig 1 Fig 2
Plain and contrast enhanced CT scans of the thorax reveals an ill defined opacity in relation to the left lower lobe. .The lesion shows early enhancement in the arterial phase. “Coning” of the aorta is seen at the level of lesion and suggests that the lesion may be supplied by the descending thoracic aorta.
Fig 3 Fig 4
Fig5 Fig6
A descending thoracic aortogram reveals hypervascularity in left lower posterior basal segment with a prominent feeding vessel arising from the left lateral aspect of lower descending thoracic aorta subsequently draining into the left pulmonary vein.These findings are suggestive of intralobar bronchopulmonary sequestration.
Fig 7 Fig 8
The pulmonary angiogram showed no arterial branch to or venous drainage from the left lower lobe.
Fig 9 Fig 10
Discussion
Pulmonary sequestration is an embryonic mass of lung tissue that has no bronchial communication and that receives its blood supply from systemic arteries.Bronchopulmonary sequestrations are classified as
1) Extralobar sequestration (EL
S) 2) Intralobar sequestration (ILS). ELS is the nonfunctioning primitive pulmonary parenchymal tissue which has no connection with the tracheobronchial tree. It consists of dilated bronchioles, alveolar ducts, and alveoli, which form the bulk of the lesion. The interstitium is composed of connective tissue, the thickness of which is depending upon the age of patient. This type of sequestration is called extralobar because the mass lies outside of the
normal investment of visceral pleura; it also may lie outside of the thorax in subdiaphragmatic region. ELS is widely accepted to be congenital in origin The accepted theory is that ELS arises as an accessory lung bud that develops from the ventral aspect of the primitive foregut. The accessory lung bud migrates caudally with the foregut and receives its blood supply from the splanchnic plexus – just as the foregut does. If the bud arises after the pleure have developed, it is not incorporated within the lung visceral pleura, and an ELS is formed. ELS is associated with other congenital abnormalities. The most common association is with diaphragmatic hernias (20%); others include – congenital cystic adenomatoid malformation (CCAM), bronchogenic cysts, and foregut malformations. ILS is also a nonfunctioning area of pulmonary parenchyma and usually is not in communication with the tracheobronchial tree; however, it may contain air via the pores of Kohn or a connection to normal small bronchi. ILS is incorporated within the normal visceral pleura of the lung, An ILS, when discovered, usually contains dense fibrous parenchyma,
which has replaced the normal pulmonary tissue as the result of chronic inflammation and fibrosis. Multiple cysts are noted which contain viscid fluid. The pleura is thickened by adhesions to mediastinal and diaphragmatic parietal pleura. Remnants of bronchi and bronchioles are replaced by fibrous connective tissue containing inflammatory infiltrates, as are alveolar ducts and alveoli. The current widely accepted theory hold that ILS is acquired – after one or more episodes of necrotizing pneumonia resulting in obliterative bronchitis and obstruction of a lower
lobe bronchus. This phase is followed by interruption of the pulmonary arterial supply to the infected lung parenchyma and hypertrophy of the systemic arterial supply from the thoracic aorta,. The diaphragmatic pleural supply involves the celiac axis aorta and abdominal aorta, and these vessels also may be recruited. Venous drainage remains via the pulmonary veins. .
Clinical presentation:Extralobar sequestration: A majority patients present in the first six months of life. Antenatal USG showing maternal polyhydramnios, fetal ascites and hydrothorax may indicate the diagnosis. On the first day of life, patients can present with dyspnea, cyanosis, and feeding difficulties. In addition, patients with ELS may present with recurrent chest infections, Symptoms can occur as a result of other associated anomalies, Intralobar sequestration Patients generally present with respiratory tract infection. Symptoms may occur from associated
anomalies. The most common anomalies associated with ILS are esophagobronchial fistule and diverticula, implying the presence of a bronchopulmonary foregut malformation. Physical examination may reveal signs of pulmonary consolidation. Auscultation may identify a bruit or continuous murmur over the sequestered lung segment from a large systemic blood supply.Radiographic findings vary depending upon:
1) Size of the lesion
2) Whether the lesion is infected.
3) The presence or absence of communication with an airway
4)The presence of associated anomalies. An uninfected sequestration is seen as a well-defined mass or as a cyst in the medial aspect of lung bases. An infected sequestration appears ill defined, and may contain fluid levels. Large sequestrations may present with an opaque hemithorax, with or without ipsilateral effusion. Intralobar sequestration appears as a mass, cystic lesion, or infiltrative shadow with ill-defined borders. Extralobar sequestrations are small lesions and are not visible on chest radiographs. However, they may present as an infiltrate or mass in the region between the lower lobes The vascular supply and venous drainage of both ILS and ELS lesions can be defined with CT scans. CT scan also provide information regarding the morphologic structure and attenuation values of any focus. In ELS, 80% of sequestrations lie between the lower lobe and the diaphragm. Lesions are usually located in the region of the posterior basal segments of the lower lobes. .In ILS, sequestrations occur within pulmonary visceral pleurae and do not communicate with the bronchial tree. Lesions of ILS may be solid, fluid, hemorrhagic, or mucous
containing cystic elements may be present, and t collapse of adjacent lung may be noted.. Most lesions appear hypervascular. Super-added infection may lead to consolidation in adjacent segments. Mucoid impaction of a bronchus surrounded by hyperinflated lung is believed to be characteristic of ILS. Angiography:-: Pulmonary sequestrations are mainly supplied from thoracic or abdominal aorta. In the remaining cases the blood flow may be from the subclavian, intercostal, pulmonary, pericardiophrenic, innominate or internal mammary arteries, The arterial supply typically enters the lung via the pulmonary ligament if the artery originates above the diaphragm. Arteries originating below the diaphragm reach the sequestration by piercing the diaphragm or via the aortic or esophageal hiatus. The arterial supply usually is composed of a dilated single vessel. This vessel is typically
0.5-2.0 cm in diameter and multiple arteries are present in some cases in which the arteries are 3 mm or smaller in diameter. Venous drainage occurs most often via the pulmonary vein in ILS – thus establishing a left-to-right shunt and the drainage occurs via bronchial or other systemic veins in ELS. Occasionally, drainage is solely to the azygos or hemiazygos systemic veins.
CASE OF THE MONTH – JUNE 2006
CASE CONTRIBUTED BY Dr. Gitanjali Bajaj
Clinical profile
A thirty-year-old male presented with complaints of painful swelling in the left shoulder region with weakness of all the four limbs.
Radiological Findings:
A plain radiograph of the left shoulder revealed an expansile, trabeculated, lytic lesion arising from the superolateral aspect of the left scapula The lesion produced bulging and thinning of the cortex and had a narrow zone of transition . No periosteal reaction was seen. The glenoid articular surface appeared to be intact. (Fig 1)
Fig.1
The differential diagnosis considered were : Giant cell tumor, metastasis and solitary plasmacytoma.
Computed tomography
A CT scan revealed an expansile lesion with cortical thinning associated with a soft tissue Mass (Fig 2, Fig 3).
Fig.2
Fig.3
MRI showed a large, multilobulated, expansile lesion in the superolateral aspect of the left scapula involving the coracoid process . It appeared isointense on T1W images, hyperintense on T2WI and shows homogenous postcontrast enhancement. It showed break of the cortex with extensive involvement of the subscapularis and the
supraspinatus muscles. Multiple flow voids were seen within it suggestive of tumoral vascularity. Rest of the scapula was normal.
Hyperintense lesion seen within the rotator cuff muscles, represented tumoral infiltration/ lymphatic edema. Minimal fluid was seen in the glenohumeral joint and along the biceps tendon. The glenohumeral joint was normal. (Figs 4-9)
Fig. 4 Transverse T1-weighted spin echo Fig. 5 Transverse T2-weighted spin echo image
Fig. 6 Coronal T1-weighted spin echo Fig. 7 Post-contrast coronal T1 Weighted spin echo
Fig. 8 Sagittal proton density fat saturation image Fig. 9 Sagittal TIRM image
Other investigations
Blood investigations showed normal hemoglobin, erythrocyte sedimentation rate (ESR), fasting and postprandial blood sugar, liver and renal profile. Urine examination for Bence-Jones proteins and immunoelectrophoresis was negative. Serum protein electrophoresis was normal, but an M band (IgG, l light chain) was seen
on immunoelectrophoresis. Skeletal survey was negative – radiographs of the skull, spine, ribs and pelvis were
normal. A CT scan of chest, abdomen and pelvis revealed no other lytic lesions. EMG studies were consistent with features of peripheral neuropathy. A core biopsy of the lesion revealed atypical plasma cells; whereas bone marrow biopsy showed 1% plasma cells with no evidence of plasma cell dyscrasia..
Final Diagnosis : Solitary bone plasmacytoma .
This case shows a rare association of solitary bone plasmacytoma with peripheral neuropathy.
Discussion
Solitary plasmacytomas are tumors composed of monoclonal plasma cells, which are cytologically, immunophenotypically, and genetically identical to those seen in multiple myeloma, but occur as solitary lesions. Solitary plasmacytomas can be divided into two groups according to the location. If the single tumor originates in the bone marrow , it is solitary bone plasmacytoma. If it arises from the tissues other than bone marrow it is called extramedullary plasmacytoma. Most of these are found in upper air passages and oral cavity.
Incidence : Solitary plasmacytomas as compared to multipla myeloma are rare representing less than 5 % of the plasma cell dyscrasias.
Age sex distribution :
It mainly affects younger patients. As many as 25% of the patients with plasmacytoma are 30 years of age or younger. It is more common in males . M: F = 4:1.
Location :
Solitary bone plasmacytoma tends to involve the axial skeleton and spares the skull and long bones. Over 40% of solitary plasmacytomas are localized in the vertebrae (thoracic spine > lumbar spine ) followed by pelvis , skull, sternum, ribs and scapula. It is interesting to note that the ribs, which are frequent sites in multiple myeloma , are not initially involved in the solitary lesion.
Presentation :
Most patients present with pain secondary to bone destruction by the infiltraring plasma cell tumor. It is commonly accompanied by neurological manifestations , detected in as many as 25 % of cases of plasmacytoma .
A rare presentation of a solitary plasmacytoma is the POEMS syndrome: Polyneuropathy, Organomegaly, Endocrinologic abnormalities (amenorrhea, impotence, diabetes, etc.), M-protein, and Skin changes. It is thought to be caused by cytokines produced by the tumor.
Imaging findings :
Plain radiograph: A solitary growth tends to be larger and more expansile than any individual lesion of multiple disease and is frequently complicated by a pathological fracture.
The spine : A bubbly expansile lytic lesion in the vertebral body causing bulging and thinning of the anterior and posterior cortices. It may resemble the appearance of an aneurysmal bone cyst. Eventually there is cortical penetration and vertebral body collapse.
The pelvis : An osteolytic lesion in the pelvis may take any of the following appearances::
(1) A sharply circumscribed area of rarefaction with or without a sclerotic border ;
(2) Dissolution of a localized segment of cancellous and cortical bone ;
(3) A giant septated lytic lesion with a lobulated sclerotic margin;
(4) A diffuse loss of cancellous bone structure in the entire bone ; or
(5) A generalized miliary type of dissemination throughout the entire pelvis.
The differential diagnosis of these lesions include simple bone cyst , giant cell tumor and metastasis.
The skull : Solitary myeloma of the skull may present as single large area of osteolysis resembling osteoporosis circumscripta or as an expansile multiloculated lytic bony defect involving the inner and outer tables of the skull. It is similar to lesions seen in metastases of thyroid or kidney origin.
Computed tomography :. CT can be used to detect the extent of osseous and soft tissue involvement – especially in areas of complex anatomy such as the spine , shoulder and pelvis.
Magnetic Resonance imaging:
The MRI appearance is consistent with that of a focal area of bone marrow replacement; The signal intensity is similar to muscle on T1-weighted images and hyperintense relative to muscle on T2-weighted images. It tends to enhance somewhat with gadolinium administration. An extraosseous soft-tissue component is often present.
Short tau inversion recovery (STIR) sequence may allow detection of small focal collections of tumor that escape visualization with standard spin echo MR imaging methods.
Diagnosis :
The distinction between multiple myeloma and solitary bone plasmacytoma is important as therapy for solitary bone plasmacytoma is definitive local radiotherapy whereas therapy for multiple myeloma is systemic and includes steroids, irradiation, and chemotherapy. Criteria for diagnosis of Solitary Bone Plasmacytoma
1. Single bone lesion – as demonstrated on complete radiographic skeletal survey and CT/MRI scan of the axial skeleton ( skull, spine, pelvis, proximal femora and humeri)
2. Clonal plasmacytosis – seen on the biopsy of the tumor or flow cytometry and immunohistochemistry.
3. Normal bone marrow – lack of clonal plasma cells or aneuploidy on flow cytometry.
4. Absent or low, serum or urinary levels of monoclonal proteins- if present at the time of diagnosis should disappear after 6 – 12 months of therapy.
5. Preserved levels of uninvolved immunoglobulins.
6. No anemia, hypercalcemia or renal impairment attributable to myeloma.
Treatment
The standard treatment for a solitary bone plasmacytoma is irradiation to the entire lesion with appropriate margins. The dose used in radiation therapy for solitary bone plasmacytoma is typically between 3,000 and 4,500 cGy. Treatment with radiation is associated with cure in approximately 40-50% of patients without evidence of recurrence 10 years after treatment. Both osseous and extraosseous or extramedullary plasmacytomas are treated with radiation therapy. Surgical resection is rarely necessary Adjuvant chemotherapy has been administered with inconclusive results. Although some studies have found that adjuvant therapy may prevent or delay progression to myeloma, most have noted no benefit with early administration of chemotherapy.
Prognosis:
While the majority of patients with solitary bone plasmacytoma develop myeloma after a median of 2–3 years, the overall median survival of 7–12 years is longer than for patients in early phases of symptomatic myeloma. Approximately 15%–45% of patients remain disease free at 10 years and although the majority of these appear to be cured, rare late recurrences have been reported. Hence, after completion of radiotherapy, patients should be monitored regularly. The monitoring should include serum and urine immunofixation, complete blood count,
serum calcium, and creatinine every 4 to 6 months for one year, and annually thereafter. Patients should also receive a bone survey or MRI annually or sooner if the patient develops a serum M protein after treatment or an increase in a persistent M protein.
Summary :
Solitary bone plasmacytoma (SBP) is a rare presentation of plasma cell neoplasms. It’s association with peripheral neuropathy is all the more rare. In contrast to multiple myeloma, long-term disease-free survival and cure is possible with local radiotherapy (RT). Imaging alone cannot differentiate these tumors from more common malignant
entities such as carcinoma, meningioma in cases of intracranial extramedullary plasmacytomas or metastasis from other primaries. The role of imaging should be focused on early detection of additional or recurrent lesions and the presence of regional lymphadenopathy which will influence clinical management.
Case of the month May 2006
Case contributed by Dr. Alok Sao
Clinical profile
A nine-month-old girl was brought with complaints of recurrent upper respiratory tract infection since the age of two months. She had history of regurgitation after feeds.
Radiological Findings:
Plain radiographs of the chest revealed a well-defined soft tissue opacity in the right lower zone in the paracardiac region. Laterally, the margin of the soft tissue opacity could be traced upto the hilum. Inferiorly, the lesion seemed to extend beyond the diaphragm. The thoraco-abdominal sign was positive and the medial border of the opacity could not be well delineated. The right border of heart could be seen well through the soft tissue opacity which was thus interpreted to be posterior placed possibly arising from below the diaphragm. .
Fig.1 Fig.2
A CT scan revealed herniation of the stomach posterior to the heart in the right paraspinal region.
Fig.3 Fig.4
A barium study confirmed the diagnosis of herniation of stomach into the thoracic cavity posterior to the heart on the right side.
Fig.5 Fig.6
Discussion
A congenital diaphragmatic hernia (CDH) is a displacement of abdominal contents into the thoracic cavity through a defect in the diaphragm. Riverius, who incidentally noted a CDH during a postmortem examination of a 24-year-old person, first described CDH in 1679.
CDH occurs 1 in every 2000-4000 live births and accounts for 8% of all major congenital anomalies.
Benjamin et al reported a male preponderance in left-sided hernias – with a male-to-female ratio of 3:2. The incidence is even more striking in right-sided hernias, with a male-to-female ratio of 3:1. The risk of recurrence of isolated CDH for future siblings is approximately 2%.
Age: While CDH is most commonly a disorder of the newborn period, as many as 10% of patients may present after the newborn period and even during adulthood. Outcome in patients with late presentation of CDH is extremely good with low or no mortality.
Pathophysiology:
The three basic types of CDH are the posterolateral Bochdalek hernia (occurring at approximately 6 weeks’ gestation), the anterior Morgagni hernia, and the hiatus hernia. The left-sided Bochdalek hernia occurs in approximately 90% of cases. Left-sided hernias allow herniation of both small and large bowel as well as intra-abdominal solid organs into the thoracic cavity. In right-sided hernias, only the liver and a portion of the large bowel tend to herniate. Bilateral hernias are uncommon and usually fatal. The major problem in a Bochdalek hernia is the posterolateral defect of the diaphragm which results in either the failure of the pleuro-peritoneal folds to develop or the improper or absent migration of the diaphragmatic musculature. Bilateral Bochdalek hernias are rare.
The Morgagni hernia is a less-common CDH, occurring in only 5-10% of cases of CDH. This hernia occurs in the anterior midline through the sternocostal hiatus of the diaphragm, with 90% of cases occurring on the right side.
CDH is characterized by a variable degree of pulmonary hypoplasia associated with a decrease in the cross-sectional area of the pulmonary vasculature and dysfunction of the surfactant system.
Associations: De Lange syndrome, Fryns syndrome, Trisomy 13, Trisomy 18
Clinical presentation:
Infants may have an antenatal history of polyhydramnios. Infants most commonly present with a history of cyanosis and respiratory distress in the first minutes or hours of life – although a later presentation is possible. Frequently, infants exhibit a scaphoid abdomen, respiratory distress and cyanosis. In left-sided posterolateral hernias, auscultation of the lungs reveals poor air entry on the left with a shift of cardiac sounds over the right chest.
Morgagni hernias are usually asymptomatic in the infant.
Imaging :
Antenatal USG- In patients presenting in the prenatal period, ultrasonographic features indicative of CDH include the following: polyhydramnios; an absent or intrathoracic stomach bubble; mediastinal and cardiac shift away from the side of the herniation.
Chest radiograph – Typical findings in left-sided posterolateral CDH include air- or fluid-filled loops of the bowel in the left hemithorax and shift of the cardiac silhouette to the right.
CT scan usually reveals retroperitoneal fat, kidney and bowel loops herniating through the defect.
Barium studies reveals herniation of bowel loops , stomach through the defect.
Treatment :
Until recently, specialists believed that reduction of the herniated viscera and closure of the diaphragmatic defect should be performed emergently following birth. More recent research demonstrates that a delayed surgical approach that enables preoperative stabilization decreases morbidity and mortality. This change is due to the recent understanding that pulmonary hypoplasia, PPHN, and surfactant deficiency are largely responsible for the outcome of CDH and that the severity of these pathophysiologies is largely predetermined in utero. The pathophysiology does not appear to be exacerbated postnatally by herniated viscera in the chest as long as bowel decompression is continuous using a nasogastric tube.
Case of the month May 2006
Case contributed by Dr. Alok Sao
Clinical profile
A nine-month-old girl was brought with complaints of recurrent upper respiratory tract infection since the age of two months. She had history of regurgitation after feeds.
Radiological Findings:
Plain radiographs of the chest revealed a well-defined soft tissue opacity in the right lower zone in the paracardiac region. Laterally, the margin of the soft tissue opacity could be traced upto the hilum. Inferiorly, the lesion seemed to extend beyond the diaphragm. The thoraco-abdominal sign was positive and the medial border of the opacity could not be well delineated. The right border of heart could be seen well through the soft tissue opacity which was thus interpreted to be posterior placed possibly arising from below the diaphragm. .
Fig.1 Fig.2
A CT scan revealed herniation of the stomach posterior to the heart in the right paraspinal region.
Fig.3 Fig.4
A barium study confirmed the diagnosis of herniation of stomach into the thoracic cavity posterior to the heart on the right side.
Fig.5 Fig.6
Discussion
A congenital diaphragmatic hernia (CDH) is a displacement of abdominal contents into the thoracic cavity through a defect in the diaphragm. Riverius, who incidentally noted a CDH during a postmortem examination of a 24-year-old person, first described CDH in 1679.
CDH occurs 1 in every 2000-4000 live births and accounts for 8% of all major congenital anomalies.
Benjamin et al reported a male preponderance in left-sided hernias – with a male-to-female ratio of 3:2. The incidence is even more striking in right-sided hernias, with a male-to-female ratio of 3:1. The risk of recurrence of isolated CDH for future siblings is approximately 2%.
Age: While CDH is most commonly a disorder of the newborn period, as many as 10% of patients may present after the newborn period and even during adulthood. Outcome in patients with late presentation of CDH is extremely good with low or no mortality.
Pathophysiology:
The three basic types of CDH are the posterolateral Bochdalek hernia (occurring at approximately 6 weeks’ gestation), the anterior Morgagni hernia, and the hiatus hernia. The left-sided Bochdalek hernia occurs in approximately 90% of cases. Left-sided hernias allow herniation of both small and large bowel as well as intra-abdominal solid organs into the thoracic cavity. In right-sided hernias, only the liver and a portion of the large bowel tend to herniate. Bilateral hernias are uncommon and usually fatal. The major problem in a Bochdalek hernia is the posterolateral defect of the diaphragm which results in either the failure of the pleuro-peritoneal folds to develop or the improper or absent migration of the diaphragmatic musculature. Bilateral Bochdalek hernias are rare.
The Morgagni hernia is a less-common CDH, occurring in only 5-10% of cases of CDH. This hernia occurs in the anterior midline through the sternocostal hiatus of the diaphragm, with 90% of cases occurring on the right side.
CDH is characterized by a variable degree of pulmonary hypoplasia associated with a decrease in the cross-sectional area of the pulmonary vasculature and dysfunction of the surfactant system.
Associations: De Lange syndrome, Fryns syndrome, Trisomy 13, Trisomy 18
Clinical presentation:
Infants may have an antenatal history of polyhydramnios. Infants most commonly present with a history of cyanosis and respiratory distress in the first minutes or hours of life – although a later presentation is possible. Frequently, infants exhibit a scaphoid abdomen, respiratory distress and cyanosis. In left-sided posterolateral hernias, auscultation of the lungs reveals poor air entry on the left with a shift of cardiac sounds over the right chest.
Morgagni hernias are usually asymptomatic in the infant.
Imaging :
Antenatal USG- In patients presenting in the prenatal period, ultrasonographic features indicative of CDH include the following: polyhydramnios; an absent or intrathoracic stomach bubble; mediastinal and cardiac shift away from the side of the herniation.
Chest radiograph – Typical findings in left-sided posterolateral CDH include air- or fluid-filled loops of the bowel in the left hemithorax and shift of the cardiac silhouette to the right.
CT scan usually reveals retroperitoneal fat, kidney and bowel loops herniating through the defect.
Barium studies reveals herniation of bowel loops , stomach through the defect.
Treatment :
Until recently, specialists believed that reduction of the herniated viscera and closure of the diaphragmatic defect should be performed emergently following birth. More recent research demonstrates that a delayed surgical approach that enables preoperative stabilization decreases morbidity and mortality. This change is due to the recent understanding that pulmonary hypoplasia, PPHN, and surfactant deficiency are largely responsible for the outcome of CDH and that the severity of these pathophysiologies is largely predetermined in utero. The pathophysiology does not appear to be exacerbated postnatally by herniated viscera in the chest as long as bowel decompression is continuous using a nasogastric tube.
Case of the month April 2006
Case contributed by Dr. Girish Yenvankar
Clinical profile
A 29-yeaar-old lady presented with dysphagia since two years. The dysphagia was more for solids than liquids and was progressively increasing. There was no history of vomiting, weight loss or fever.
Radiological Findings –
A plain radiograph of the chest showed a soft tissue opacity in the right paravertebral region with an air-fluid level in the upper dorsal region This suggested a dilated esophagus(Fig 1).
Fig 1
A CT scan of the chest confirmed this. There was narrowing at the lower end of the esophagus. No soft tissue mass was seen in the region of the narrowing (Figs 2,3,4)
Fig 2,3,4
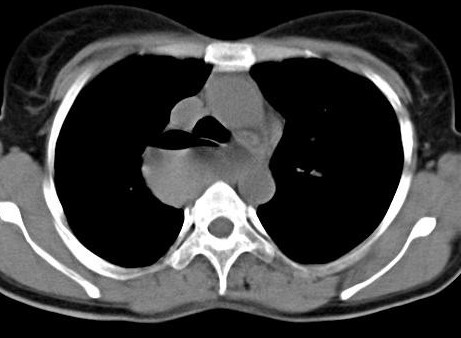
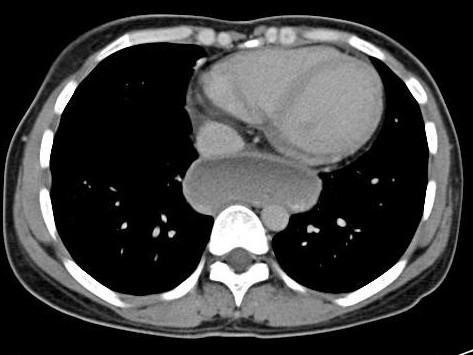
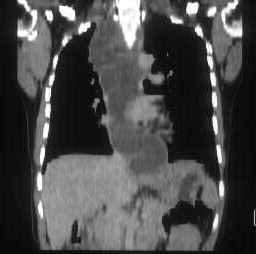
A barium esophagogram a showed smooth narrowing at the gastroesophageal junction extending for a distance of about 2 cms. with moderate dilatation of the proximal esophagus. A bird-beak appearance is seen at the distal portion of the esophagus with no evidence of shouldering. The esophageal mucosal pattern was normal. It showed no intrinsic or extrinsic filling defects or mass effect. Tertiary contractions were seen in the proximal esophagus. There was no evidence of hiatal hernia or gastro-esophageal reflux.
Figs 5,6,7,8
The patient was diagnosed to have achalasia cardia.
Discussion
Dysphagia is the most common presenting symptom in patients with achalasia. The ingestion of either solids or liquids can result in dysphagia, though dysphagia for solids is more common. The natural history varies. Some patients notice that the dysphagia reaches a certain point of severity and then stops progressing. In others, the dysphagia continues to worsen, resulting in decreased oral intake and malnutrition. Therefore, weight loss is included in the complex of signs and symptoms associated with achalasia, and it is usually a sign of advanced esophageal disease. Some of patients with dysphagia complain of episodes of chest pain which are frequently induced by eating. Typically, chest pain is described as being retrosternal; this is a more common feature in patients with early or so-called vigorous achalasia. As the disease progresses and as the esophageal musculature fails, chest pain tends to abate or disappear. Many of patients with achalasia experience spontaneous regurgitation of undigested food from the esophagus during the course of the disease. Some learn to induce regurgitation to relieve the retrosternal discomfort related to the distended esophagus. As the disease progresses the likelihood that aspiration will occur increases. As a result, some patients may present with signs or symptoms of pneumonia or pneumonitis. Lung abscesses, bronchiectasis, and hemoptysis are some of the more severe pulmonary consequences of achalasia-associated aspiration.
Pathophysiology:
The exact etiology of achalasia is not known. The most widely accepted current theories implicate autoimmune disorders, infectious diseases, or both. The last decade has witnessed much progress in the understanding of the cellular and molecular derangements in achalasia. Degeneration of the esophageal myenteric plexus of Auerbach is the primary histologic finding.
Radiologcial Studies –
Plain radiograph -Findings: Plain chest radiographs occasionally offer clues in the diagnosis of achalasia. A double
mediastinal stripe is occasionally depicted. An air-fluid level can be seen in the esophagus; this is frequently retrocardiac. Owing to the paucity of air progressing through the hypertensive LES, the gastric air bubble may be small or absent.
Barium Swallow-
Features of achalasia depicted at barium study under fluoroscopic guidance include the following: Failure of peristalsis to clear the esophagus of barium with the patient in the recumbent position Antegrade and retrograde motion of barium in the esophagus secondary to uncoordinated, nonpropulsive, tertiary contractions
Pooling or stasis of barium in the esophagus when the esophagus has become atonic or noncontractile (which occurs late in the course of disease)
LES relaxation that is incomplete and not coordinated with esophageal contraction
Dilation of the esophageal body, which is typically maximal in the distal esophagus Tapering of the barium column at the unrelaxed LES, resulting in the bird beak sign Associated epiphrenic diverticula sometimes seen.
CT scan –CT scanning with oral contrast enhancement may demonstrate the gross structural esophageal abnormalities associated with achalasia, especially dilatation, which is seen in advanced stages. However, CT findings are nonspecific, and the diagnosis of achalasia cannot be made using CT alone. CT scan may be indicated in the workup of patients with suspected pseudoachalasia.
Treatment: .
Pharmacologic therapy for achalasia: Calcium channel blockers – Nifedipine and verapamil ,Anticholinergic agents – Cimetropium bromide ,Nitrates – Isosorbide dinitrate ,Opioids – Loperamide Pneumatic Balloon Dilatation -Mechanical therapy for achalasia consists of esophageal dilation, the object of which is to disrupt muscle fibers of the LES, effecting a decrease in LES pressure. Dilation is most commonly performed by using pneumatic balloons.
Botulinum toxin (Botox) therapy –This is new modality of treatment Esophageal (Heller) myotomy is a surgical procedure that is now commonly performed with minimally invasive techniques. The laparoscopic approach appears to be most appropriate.
CASE OF THE MONTH MARCH 2006:
Contributed by Dr. Yogeshwari Deshmukh
Clinical history
A 22-year-old man presented with fullness of abdomen associated with intermittent episodes of vomiting since six months. Except for his asthenic built, there was no positive physical finding on examination. Routine laboratory investigations were within normal limits.
Radiological examination:
An upper GI series revealed dilatation of the first and second parts of the duodenum with an abrupt vertical cut off at its third part. The mucosal pattern was normal. The obstruction to passage of barium was dramatically relieved in the lateral decubitus position
Fig.1, 2
A contract enhanced CT scan of the abdomen showed a dilated proximal duodenum with abrupt cut off at its third part. The superior mesenteric artery (SMA) was seen to pass anterior to the site of the cut off. This was associated with a narrowed aorta-SMA distance.
Fig.3, 4.
These radiological findings were interpreted as a manifestation of the superior mesenteric artery syndrome.
The patient was operated. Intraoperative findings confirmed the diagnosis of SMA syndrome as the third part of duodenum was seen to be compressed between the aorta and SMA. A Roux-en-Y jejunostomy was performed.
Discussion
The SMA syndrome is an uncommon, but well recognized, clinical entity characterized by compression of the third or transverse portion of the duodenum against the aorta by the SMA – resulting in chronic, intermittent or acute – complete or partial duodenal obstruction. The SMA syndrome was first described in 1861 by Von Rokitansky, who
proposed that its cause was obstruction of the third part of the duodenum as a result of arteriomesenteric compression.
Clinical Presentation:
Patients often present with chronic upper abdominal symptoms such as epigastric pain, nausea, eructation, voluminous vomiting (bilious or partially digested food), postprandial discomfort, early satiety, and sometimes – sub acute small-bowel obstruction. The symptoms are typically relieved when the patient is in the left lateral decubitus, prone or knee-to-chest position and they are often aggravated when the patient is in the supine position. An asthenic habitus is noted in about 80% of cases. Abdominal examination may reveal a succussion splash.
Pathophysiology:
The SMA usually forms an angle of approximately 45° (range, 38-56°) with the abdominal aorta. The third part of the duodenum crosses caudal to the origin of the SMA coursing between the SMA and the aorta. Any factor that sharply narrows the aortomesenteric angle to approximately 6-25° can cause entrapment and compression of
the third part of the duodenum as it passes between the SMA and the aorta – resulting in the SMA syndrome. In addition, the aortomesenteric distance in SMA syndrome is decreased to 2-8 mm (normal is 10-20 mm). Alternatively, other causes implicated in the SMA syndrome include high insertion of the duodenum at the ligament of Treitz, a low origin of the SMA and compression of the duodenum due to peritoneal adhesions. The important etiologic factors that may precipitate a narrowing of the aortomesenteric angle and result in chronically recurrent mechanical obstruction include the following:
Constitutional factors
Thin body build
Exaggerated lumbar lordosis
Visceroptosis and abdominal wall laxity
Depletion of the mesenteric fat caused by rapid severe weight loss due to catabolic states such as cancer and burns
Severe injuries, such as head trauma, leading to prolonged bed rest
Dietary disorders: Anorexia nervosa, Malabsorption
Spinal disease, deformity, or trauma
Use of body cast in the treatment of scoliosis or vertebral fractures
Anatomic anomalies –
Abnormally high and fixed position of the ligament of Treitz with an upward displacement of the duodenum
Unusually low origin of the SMA
Imaging Studies:
The diagnosis of SMA syndrome is difficult. Confirmation usually requires radiographic studies such as an upper GI series, hypotonic duodenography and CT scanning.
An uipper GI study with barium reveals characteristic dilatation of the first and second parts of the duodenum with an abrupt vertical or linear cutoff in the third part with normal mucosal folds. Fluoroscopy demonstrates a to-and-fro motion of the barium in the dilated proximal portion of the duodenum. Other findings include a delay of 4-6 hours in gastroduodenal transit and relief of the obstruction when the patient is in the left lateral decubitus position. A Hayes maneuver (i.e. pressure applied below the umbilicus in cephalad and dorsal direction), which elevates the root of small-bowel mesentery may also relieve the obstruction.
CT scanning is useful in the diagnosis of the SMA syndrome and can provide diagnostic information including the aorta-SMA distances and duodenal distension. Also, it can be used to assess intra-abdominal and retroperitoneal fat.
Upper GI endoscopy may be necessary to exclude mechanical causes of duodenal obstruction. However, the diagnosis of SMA syndrome may be missed with this study.
Abdominal Ultrasonography may be helpful in measuring the angle of the SMA and the aortomesenteric distance.
Treatment:
Medical Care: Reversing or removing the precipitating factor is usually successful in a patient with acute SMA syndrome. Conservative initial treatment is recommended in all patients with the SMA syndrome; this includes adequate nutrition, GI decompression, and proper positioning of the patient after eating (i.e. left lateral decubitus, prone, or knee-to-chest position).
Surgical Care: Surgical intervention is indicated when conservative measures are ineffective, particularly in patients with a long history of progressive weight loss, pronounced duodenal dilatation with stasis and complicating peptic ulcer disease. Duodenojejunostomy is the most frequently used procedure and it is successful in about 90% of cases. The use of laparoscopic surgery that involves lysis of the ligament of Treitz and mobilization of the duodenum has also been reported.
February 2006
Case contributed by Dr.Manish Shinde
A seven-year-old girl presented with complaints of fever and joint pains since one month. She gave history of vague abdominal pain since one month. Since then too, there was a progressively increasing swelling on the vertex of the skull. On examination, this swelling had a firm consistency and was not well defined. The patient’s vital parameters were normal.
RADIOLOGICAL EXAMINATION
A radiograph of the abdomen was unremarkable.
Ultrasonography of the abdomen revealed a well-defined, hypoechoic mass measuring 6x4x4 cms in the left adrenal fossa on the upper pole of the left kidney displacing the kidney inferiorly. It was hypo-vascular with fine calcification within.
Fig 1 Fig2
A plain and contrast enhanced CT scan of the abdomen revealed a well defined 6x4x4 cms hypo dense, hypo vascular mass lesion involving the left adrenal gland with displacement of the kidney inferiorly and laterally. No surrounding infiltration was noted. Fine calcification was seen within the mass.
Fig 3 Fig 4
The skull film showed a destructive lesion with a sunray periosteal reaction over the left parietal bone.
Fig 5 Fig 6
Sonography of the skull confirmed the sunray periosteal reaction and showed a subgaleal hypoechoic soft tissue mass measuring 11x9x 8 cm.
Fig 7
A CT scan of the skull revealed lytic lesions in the left parietal bone with sunray periosteal reaction with subgaleal mass with underlying dural involvement suggestive of a metastatic involvement of the calvarium and dura.
Fig 8 Fig 9
A bone marrow smear was performed which showed atypical round cells suggestive of a neural crest tumour metastases.
MIBG scan showed increased I131 MIBG concentration within marrow cavity of the skull and spine indicating metastases.
Bone scan revealed metastases to the skull and spine.
Final diagnosis – Left neuroblastoma with metastases. Stage 4 INSS staging.
Discussion
Neuroblastomas are the commonest extracranial tumour in children and account for 6-8 % of pediatric malignancies. They originate from the cells of the neural crest origin which give rise to the sympathetic nervous system and adrenal medulla. The median age of patient at the time of diagnosis is two years but the tumour can present at any pediatric age.
Two thirds of patients with neuroblastoma have metastasis (Stage IV disease) at the time of presentation. Metastasis is commonly to the liver, spine, skull and lymph nodes. Staging is done based on the International Neuroblastoma Staging System (INSS) from stage 1 to 4S based on radiological findings, surgical resectability, lymph node involvement and bone marrow involvement.
Technetium 99m methylene diphosphonate whole body bone scintigraphy is a must for detection of metastases. MIBG scan, though less sensitive than MDP should also be done as it can detect both the primary and metastases. FDG PET is likely to play a larger role in neuroblastoma imaging in future.
All patients older than one year with stage IV tumors are considered to be in the high-risk group. These patients seem to require treatment with multi-agent chemotherapy, surgery, and radiotherapy followed by consolidation with high-dose chemotherapy and peripheral blood stem cell rescue.
The five year survival rate from diagnosis is approximately 83% for infants, 55% for children aged 1-5 years, and 40% for children older than 5 years
Case of the Month – Jan 2006
Contributed by Dr. Nilesh Porwal
CLINICAL PROFILE
A 35-year-old man, a known seropositive patient, symptomatic since one month came with complaints of dull aching, non radiating pain in the right hypochondrium & in the epigastric region associated with intermittent fever. The patient had history of pulmonary tuberculosis 15 yrs back & was on antiretroviral therapy since 1 ½ years.
On physical examination, there was mild icterus with tender hepatomegaly. However no lymphadenopathy was found.
Radiological findings:
Ultrasonography of the abdomen showed multiple, well defined hypoehoeic lesions – approximately 3cm in diameter – spread through out the liver with distortion of its contour.
Fig 1.
Plain and contrast enhanced CT scans of the abdomen revealed multiple, target lesions with minimal rim enhancement, hepatomegaly, free fluid in peritoneal cavity & enlarged aorto-caval lymph nodes.
Fig 2 Fig 3.
On MRI, these lesions appear as hyperintense on both T1 & T2 weighted images. This ruled out the possibility of fungal abscesses which are hypointense on T2 as it contain manganese & iron.
Fig 4. Fig 5.
On liver biopsy, the liver was heavily infiltrated by atypical looking mononuclear cells. There was biliary ductular proliferation. Very little normal liver parenchyma is seen. Some tumor cells showed nuclear moulding. These findings were highly suggestive of a Non Hodgkin’s Lymphoma.
Fig 6.
DISCUSSION
There are three main categories of AIDS related lymphoma.
1.Imunoblastic lymphomas {60%}: High grade or diffuse histocytic lymphoma.{large cell} common in older patients.
Burkitt’s lymphoma {20%}: Small non cleaved cell lymphoma common in young patients .
Primary CNS lymphoma {20%}.
DIFFERENTIAL DIAGNOSIS:-Lesions due to metastases, Kaposi’s sarcoma, disseminated tuberculosis, bacillary angiomatosis & Pneumocystis carinii may present similar appearances.
INCIDENCE:- Non Hodgkin Lymphoma is present in 3% of HIV positive patients at the time of their diagnosis & develops in upto 20 % HIV patients during their life time.
CLINICAL PRESENTATION: – The most common symptom is painless swelling of lymph nodes in the neck, axilla and groin. 80% of patients present with advanced disease/extra nodal presentation; of these, 80% have type B symptoms at presentation like unexplained fever, night sweats, fatigue, weight loss & anorexia.
TYPE OF NHL: – Low grade, intermediate& high grade lymphoma depend upon microscopic appearance.
DIAGNOSIS :- Biopsy is necessary to confirm the diagnosis. Other lab parameters & imaging modalities are helpful to monitor & determine the spread of disease.
STAGING OF DISEASE:- Stage I: Disease located to one region; Stage II: located to two regions on the same side of the diaphragm& Stage III: Spread to both sides of the diaphragm involving one organ or area near-by spleen or lymph nodes. In stage IV, spread beyond lymphatic system, involving one or more major organ possibly bone marrow, skin.
TRATMENT OPTION :- 1} Combination of chemotherapy
2}Radiation therapy usually along with chemotherapy.
3}Bone marrow transplant in case of recurrence.
4}Immunotherapy.
In a seropositive patient these are usually combined with antiretroviral therapy.
CONCLUSION: – AIDS Clinical trial group study suggested that prognostic variables in AIDS patients closely resemble with that of non-AIDS related NHL.
Two year survival for patients with good prognosis treated with chemotherapy is 50 % as compared with 24 % for those with poor prognosis.
September 2009
Case contributed by Dr. Sachin Kumbhar
CLINICAL PROFILE
A five-year-old female child presented to our pediatric clinic with pain in both lower limbs since 20 days. There was no history of lower limb swelling or fever. The patient had been diagnosed as having ‘bilateral lower limb deep venous thrombosis’ on color Doppler examination done elsewhere and had already been started on heparin injections for the same.
On admission to our institution, the patient was referred for a color Doppler examination to look for recanalisation of the deep venous system and residual thrombosis so that heparin could be stopped.
Radiological findings
Fig 1
On color Doppler examination, the entire deep venous system of both the lower limbs was patent with normal flow and respiratory phasic variations. No filling defect or evidence of recanalaised thrombus was seen in the deep venous system.
Fig 2, Fig 3
Fig 4,Fig 5
However on B mode, well defined hypoehoic lesions were seen extending along the shafts of long bones of both lower limbs. The lesions were peripherally limited by the periosteum. They did not reveal any internal vascularity. The overlying musculature was normal. A diagnosis of large subperiosteal hematomas involving both the femora and the tibia was made. An MRI examination was performed for confirmation.
Fig 6 , Fig 7
Fig 8
MRI scan revealed lesions along the shafts of both the femora and tibia. The lesions were periosteal in location and hyperintense on both T1 and T2 weighted images suggestive of large subacute subperiosteal hematomas. No soft tissue abnormality was seen. A possibility of scurvy was suggested. Radiographs of both the lower limbs were performed.
Fig 9, Fig 10
These radiographs reveal severe osteopenia with non prominence of the trabecular pattern of the long bones with pencil thinning of the cortices. White line of Frenkel as well as Trummerfield’s zones of rarefaction are also seen.
Diagnosis: A final diagnosis of scurvy with large subperiosteal hematomas was made.
Discussion
Scurvy is a disease caused due to deficiency of vitamin C(ascorbic acid) characterized by bone disease in growing children and haemorrhagic and healing defects.
Etiopathogenesis: Ascorbic acid functions in a variety of biosynthetic pathways by accelerating hydroxylation and amidation reactions. The most clearly established function is activation of prolyl and lysyl hydroxylases from inactive precursors providing for hydroxylation of procollagen. Inadequately hydroxylated precursors cannot acquire a stable helical configuration and cannot be adequately cross linked so that they are poorly secreted from the fibroblast. Those that are secreted lack tensile strength, are more soluble and are more vulnerable to enzymatic degradation. Collagen, which normally has the highest content of hydroxyproline is the most affected protein, particularly in the blood vessels. Proline hydroxylase is also required for the formation of osteocalcin.
Skeletal changes are seen in infants and children. The primary disturbance is in the formation of osteoid matrix rather than in mineralization or calcification as occurs in rickets. In scurvy, the palisade of cartilage cells is formed as usual and is provisionally calcified. However there is insufficient production of osteoid matrix by osteoblasts. Resorption of the cartilaginous matrix then fails or slows; as a consequence there is cartilaginous overgrowth, with long spicules and plates projecting into the metaphyseal region of marrow cavity and sometimes widening of the epiphysis. The scorbutic bone yields to the stresses of weight bearing and muscle tension with bowing of the long bones of the lower legs and abnormal depression of the sternum with outward projection of the ends of the ribs.
Clinical Features:
Scurvy may occur at any age but is rare in the newborn infant. Majority of cases occur in infants 6-24 months of age. Clinical manifestations require time to develop; after a variable period of vitamin C depletion, vague symptoms of irritability, tachypnoea, digestive disturbances and loss of appetite appear. There is generalized tenderness especially noticeable in the legs when the infant is picked up or when the diaper is changed. The pain results in pseudo paralysis and the legs assume the typical ‘frog position’, in which the hips and the knees are semi flexed with the feet rotated outwards. Edematous swelling along the shafts of the legs may be present. In some cases a subperiosteal hemorrhage can be palpated at the end of the femur. Changes in the gums, most noticeable when the teeth are erupted, are characterized by bluish purple spongy swellings of the mucous membrane, usually over the upper incisors. There may be a ‘rosary’ at the costochondral junctions and a depression of the sternum. Petechial haemorrhages may occur in the skin and the mucous membranes. Low grade fever is usually present. Anemia may reflect inability to utilize iron or chronic blood loss.
Radiological findings:
Fig 12
The diagnosis of scurvy is usually based on roentgenographic changes in the long bones , especially at their distal ends. Changes are greatest as a rule in the region of the knee. In the early stages, the appearance resembles that of simple atrophy of the bone. The trabaculae of the shaft cannot be discerned, and the bone assumes a ‘ground glass’ appearance. The cortex is reduced to pencil point thinness. The white line of Frenkel , which represents the zone of well calcified cartilage can be clearly discerned as an irregular but thickened white line at the metaphysis. The epiphyseal centres of ossification also have a ground-glass appearance and are surrounded by a white ring (Wimberger’s sign). At this stage scurvy cannot be diagnosed with certainty from the radiograph unless the zone of rarefaction under the white line at the metaphysic becomes apparent. The zone of rarefaction (Trummerfeld’s zone of rarefaction) is a linear break in the bone proximal and parallel to the white line. Often, it does not traverse the shaft in its entire width and may be seen only in the lateral parts as a triangular defect. A spur (Pelkan’s spur), as a lateral prolongation of the white line may be present. Epiphyseal separation may occur along the line of destruction, with linear displacement or compression of the epiphysis against the shaft. Subperiosteal hemorrhages are not visible roentgenographically in active scurvy. During healing, however the elevated periosteum becomes calcified and the affected bone assumes a dumbbell or club shape.
This case illustrates how lack of clinical awareness of the disease can result in unindicated/misdirected radiologic investigations.
Fig 13
December 2007.
Case contributed by Dr. Pankaj Gaurkar
CLINICAL PROFILE:
A 40-year-old lady presented with complaints of left sided earache, decreased hearing on the left and pulsatile tinnitus since four months. She had no history of ear discharge. Except for mild conductive hearing loss, her physical and ENT examinations were normal.
RADIOLOGICAL INVESTIGATIONS
HRCT of the temporal bone:
A soft tissue mass was seen in relation to inferior or jugular wall of the middle ear cavity. This mass extended into the middle ear cavity by eroding through the wall of the jugular foramen. There was also erosion of the inferior aspect of the internal auditory canal and carotid canal. On post contrast images, the mass showed moderate enhancement.
(IMA 1&2)
MRI of temporal bone:
Plain and contrast enhanced MRI of the lesion skull base showed the soft tissue mass extending inferiorly into the ipsilateral para-paryngeal space. On T1 WI, the mass was hypointense. On T2WI it showed multiple flow voids. On post contrast images, there was enhancement of the soft tissue mass.
IMA 3, 4 &5
Based on these classical radiological findings a diagnosis of a glomus jugulare tumor was made.
DISCUSSION:
Glomus jugulare tumors are rare, slow-growing, hypervascular tumors that arise within the jugular foramen of the temporal bone.
INCIDENCE:
Glomus tumors occur with an estimated annual incidence of 1 case per 1.3 million people.
Although rare, glomus tumors are the most common tumor of the middle ear and are second to vestibular shwannoma as the most common tumor of the temporal bone. The female-to-male ratio is 3-6:1. Glomus jugulare tumors have also been noted to be more common on the left side, especially in females. Most tumors occur in patients aged 40-70 years, but cases have been reported in patients as young as six months and as old as 88 years.
Although most paragangliomas are sporadic, they can be familial with autosomal dominant inheritance and incomplete penetrance
PATHOPHYSIOLOGY:
Glomus bodies or paraganglia are small collections of paraganglionic tissue. They are derived from embryonic neuroepithelium in close association with the autonomic nervous system and are found in the region of the jugular bulb. Glomus jugulare tumors originate from the chief cells of the paraganglia.
CLASSIFICATION:
The Glasscock-Jackson and Fisch classifications of glomus tumors are widely used. The Fisch classification of glomus tumors is based on extension of the tumor to surrounding anatomic structures and is closely related to mortality and morbidity.
Type A tumor – Tumor limited to the middle ear cleft (glomus tympanicum)
Type B tumor – Tumor limited to the tympanomastoid area with no infralabyrinthine compartment involvement
Type C tumor – Tumor involving the infralabyrinthine compartment of the temporal bone and extending into the petrous apex
Type C1 tumor – Tumor with limited involvement of the vertical portion of the carotid canal
Type C2 tumor – Tumor invading the vertical portion of the carotid canal
Type C3 tumor – Tumor invasion of the horizontal portion of the carotid canal
Type D1 tumor – Tumor with an intracranial extension less than 2 cm in diameter
Type D2 tumor – Tumor with an intracranial extension greater than 2 cm in diameter
CLINICAL FEATURES:
Patients present with pulsatile tinnitus, conductive hearing loss, retro tympanic mass, and cranial nerve deficits. Most tumors are slow growing but locally invasive. They may have earache, vertigo and sensory- neural hearing loss. Up to 4% of the tumors are functional and produce clinically significant levels of catecholamines, norepinephrine, or dopamine with symptoms mimicking a pheochromocytoma.
RADILOGIC EXAMINATION
Plain films may ay show enlargement of the lateral jugular foramen and fossa or erosion of adjacent bone.
HRCT features.
On high-resolution CT scans of the temporal bones, expansion and erosion of the jugular foramen characterize the glomus jugulare tumor. Perhaps the earliest abnormality detectable on cross-sectional images of a glomus jugulare tumor is erosion of the lateral and anterior walls of the osseous jugular fossa on a thin-sections bone algorithm.
Progressive growth of the tumor produces the typical moth-eaten pattern of erosion of the jugular foramen and destruction of the surrounding bony labyrinth including the
caroticojugular spine. The tumor spreads along the paths of least resistance and is initially directed superiorly owing to the intrinsic weakness of this part of the jugular fossa. Subsequently, the hypotympanum, mesotympanum, and the sinus tympani are invaded. Ossicular chain destruction is common. Inferior spread of the tumor produces infiltration of the IJV and intratemporal fossa. As the tumor spreads laterally, it may destroy the facial nerve canal and infiltrate the facial nerve. On post contrast images the mass shows intense enhancement.
MRI features:
Paragangliomas typically exhibit low signal intensity T1WI and high signal intensity with T2WI. Multiple serpentine and punctate areas of signal void characterize the typical Paraganglioma with all MR sequences; these areas are variably distributed throughout the mass and are believed to represent flow voids in the larger intratumoral vessels.
The classic ‘salt-and-pepper appearance’ was originally described by Olsen et al from appearances on long TR/long TE images. The "pepper" component represents the multiple areas of signal void interspersed with the "salt" component seen as hyperintense foci (due to slow flow or hemorrhage on both short TR and long TR images. This feature is limited to paragangliomas that are greater than 1 cm in diameter and is not considered
diagnostic. In addition to providing superior definition of location, extent, and characterization of paragangliomas, MR imaging also better demonstrates tumor involvement of the ICA and IJV compared with that seen with CT.
MR imaging can depict paragangliomas that are smaller than 5 mm, whereas CT demonstrates only lesions greater than 8 mm
Angiography: It reveals a blush; most often from the ascending pharyngeal artery additionally it may involve the posterior auricular, occipital, maxillary, or internal carotid arteries.
TREATMENT:
Medical therapy: Some cases require no treatment. Often, glomus jugulare tumors are diagnosed within the sixth or seventh decade of life and can be followed by imaging and may not need surgical intervention.
Surgery: It is the treatment of choice for glomus jugulare tumors. Surgical approach
depends on the localization and extension of the tumor. Pre- surgical embolisaion of the tumor can be done. Radiation therapy and radio-surgery can also be done.
CLINICAL PROFILE
January 2008
Case contributed by Dr. Chirag Khajanchi
A 64-year-old man, hailing from Uttar Pradesh, presented with fever and acute abdominal pain radiating to the right groin. Epigastric discomfort and dyspepsia were also present. Occupational history was non-significant. On examination, the abdomen was soft. Bilateral inguinal adenopathy was found. He had no scrotal abnormalities. His symptoms improved on empiric antibiotic therapy.
RADIOLOGICAL FINDINGS :
Plain and contrast enhanced CT scans of the chest and abdomen were performed. There was a low-density tubular structure in the posterior mediastinum adjacent to the gastro-esophageal junction and to the right of the aorta. It extended from the mid thoracic level to the level below the diaphragmatic crura.
Fig 1.
CT scan abdomen plain and contrast enhanced was performed. It revealed a cystic lesion with thin wall in relation to the GE junction and the retrocardiac esophagus. Multiple small dilated hypodense non enhanching channels were seen in the retro- peritoneum, which started at the level of renal hilum, and were traced upto the spermatic
cord on both sides. Central attenuation value of these diffusely distributed channels ranged from 8 to 27 HU in retroperitoneum and from 22 to 30 HU along common iliac and external iliac vessels. Stomach was grossly distended, however no obvious mass lesion was seen in pylorus or duodenum. No adenopathy, pleural effusion or ascites was
noted.
Fig 2. Fig 3.
Fig 4. Fig 5.
Single-shot fast spin-echo MR images in multiple planes confirmed a fluid-filled tubular structure extending from mid thoracic level cranially to the level of the spermatic cords
caudally consistent with a distended thoracic duct and dilated tortuous lymphatics. No chylothorax was present.
MR images confirmed nonenhancing fluid-filled structures distributed in the retroperitoneum and pelvis consistent with diffuse lymphangiectasia. More readily apparent on MRI were superficial inguinal lymphangiectasia and mild scrotal
subcutaneous lymphangiectasia. Multiple tortous channels hyperintense on T2W and hypointense on T1W images in retroperitoneum extending from level of spermatic cords upto level of mid cisterna chylii in thorax.
Fig 6. Fig 7. Fig 8.
Fig 9. Fig 10.
The patient underwent blood tests which revealed eosinophilia.
High-power microscopic view of microfilaria of Wuchereria bancrofti was obtained from peripheral blood smear and showed presence of distinct sheath and absence of nuclei in tail (Wright-Giemsa stain).
Fig 11.
DIAGNOSIS:
Retroperitoneal Filariasis. With dilated collateral lymphatic ducts.
DISSCUSSION
Human lymphatic filariasis is caused by infections with W. bancrofti, Brugia malayi, or Brugia timori. These parasites are found in many tropical and subtropical areas of the world. The adult worms live in the lymphatics throughout the body and cause extensive lymphangiectasia by obstructing lymph flow. Filariasis is the most common cause of acquired lymphedema in the world. Interestingly, our patient had neither scrotal nor lower extremity edema. Lower chest CT revealed a tubular structure in the posterior mediastinum, coursing along the thoracic spine. Its central attenuation value ranged from 15 H in a more distended segment to 32 H in the least distended portion, which was behind the left atrium. The low-density tubular structure in the posterior mediastinum of this patient is a distended thoracic duct. The thoracic duct collects lymph from most body tissues and transmits it back into the blood stream. The duct originates in the abdomen anterior to the second lumbar vertebra at the cisterna chyli and then ascends into the thorax through the aortic hiatus of the diaphragm slightly to the right of the midline. Within the posterior mediastinum of the thorax and still coursing just ventral to the vertebral column, the thoracic duct gradually crosses the midline to the left. The duct then ascends into the root of the neck on the left side and drains into the left subclavian vein near the junction of the left internal jugular vein. Abdominal CT scan revealed low-attenuation channels distributed in the retroperitoneum and pelvis that showed no enhancement with IV contrast material. The central attenuation value ranged from 8 to 27 HU in the retroperitoneum and 22 to 30 HU in the pelvis along the iliac vessels. No discrete mass was identified associated with this diffuse abnormality. MR images clearly revealed nonenhancing confluence of prominent lymphatic ducts and vessels. Superficial inguinal lymphangiectasia and mild scrotal subcutaneous lymphangiectasia were also more readily evident on heavily T2-weighted images.
Case et al. used MRI to detect dilated lymphatic vessels in ferrets infected with B. malayi. In 1999, Blacksin et al. reported the first description of MRI findings in a human, a case of bancroftian filariasis affecting the ankle joint .Witte et al. described the potential use of MRI, particularly fat-saturated T2-weighted images, for the evaluation of the lymphatic system. Schick et al. described cystic lymph node enlargement of the neck on MRI in a patient with filariasis. This case shows the distended thoracic duct on CT as a nonenhancing low-attenuation tubular structure in the posterior mediastinum. Its recognition is made easier by knowing the anatomic origin and course of the thoracic duct. Diffuse lymphangiectasia in the retroperitoneum and pelvis appears on CT as nonenhancing low-attenuation channels along major vessels and is not associated with a discrete mass. MR images, particularly single-shot fast spin-echo images, a heavily T2-weighted pulse sequence, helped reveal the nonenhancing fluid-filled thoracic duct and prominent lymphatic ducts and vessels.
December 2007.
Case contributed by Dr. Pankaj Gaurkar
CLINICAL PROFILE:
A 40-year-old lady presented with complaints of left sided earache, decreased hearing on the left and pulsatile tinnitus since four months. She had no history of ear discharge. Except for mild conductive hearing loss, her physical and ENT examinations were normal.
RADIOLOGICAL INVESTIGATIONS
HRCT of the temporal bone:
A soft tissue mass was seen in relation to inferior or jugular wall of the middle ear cavity. This mass extended into the middle ear cavity by eroding through the wall of the jugular foramen. There was also erosion of the inferior aspect of the internal auditory canal and carotid canal. On post contrast images, the mass showed moderate enhancement.
(IMA 1&2)
MRI of temporal bone:
Plain and contrast enhanced MRI of the lesion skull base showed the soft tissue mass extending inferiorly into the ipsilateral para-paryngeal space. On T1 WI, the mass was hypointense. On T2WI it showed multiple flow voids. On post contrast images, there was enhancement of the soft tissue mass.
IMA 3, 4 &5
Based on these classical radiological findings a diagnosis of a glomus jugulare tumor was made.
DISCUSSION
Glomus jugulare tumors are rare, slow-growing, hypervascular tumors that arise within the jugular foramen of the temporal bone.
INCIDENCE:
Glomus tumors occur with an estimated annual incidence of 1 case per 1.3 million people.
Although rare, glomus tumors are the most common tumor of the middle ear and are second to vestibular shwannoma as the most common tumor of the temporal bone.
The female-to-male ratio is 3-6:1. Glomus jugulare tumors have also been noted to be more common on the left side, especially in females.
Most tumors occur in patients aged 40-70 years, but cases have been reported in patients as young as six months and as old as 88 years.
Although most paragangliomas are sporadic, they can be familial with autosomal dominant inheritance and incomplete penetrance
PATHOPHYSIOLOGY:
Glomus bodies or paraganglia are small collections of paraganglionic tissue. They are derived from embryonic neuroepithelium in close association with the autonomic nervous system and are found in the region of the jugular bulb. Glomus jugulare tumors originate from the chief cells of the paraganglia.
CLASSIFICATION:
The Glasscock-Jackson and Fisch classifications of glomus tumors are widely used. The Fisch classification of glomus tumors is based on extension of the tumor to surrounding anatomic structures and is closely related to mortality and morbidity.
Type A tumor – Tumor limited to the middle ear cleft (glomus tympanicum)
Type B tumor – Tumor limited to the tympanomastoid area with no infralabyrinthine compartment involvement
Type C tumor – Tumor involving the infralabyrinthine compartment of the temporal bone and extending into the petrous apex
Type C1 tumor – Tumor with limited involvement of the vertical portion of the carotid canal
Type C2 tumor – Tumor invading the vertical portion of the carotid canal
Type C3 tumor – Tumor invasion of the horizontal portion of the carotid canal
Type D1 tumor – Tumor with an intracranial extension less than 2 cm in diameter
Type D2 tumor – Tumor with an intracranial extension greater than 2 cm in diameter
CLINICAL FEATURES:
Patients present with pulsatile tinnitus, conductive hearing loss, retro tympanic mass, and cranial nerve deficits. Most tumors are slow growing but locally invasive. They may have earache, vertigo and sensory- neural hearing loss. Up to 4% of the tumors are functional and produce clinically significant levels of catecholamines, norepinephrine, or dopamine with symptoms mimicking a pheochromocytoma.
RADILOGIC EXAMINATION
Plain films may ay show enlargement of the lateral jugular foramen and fossa or erosion of adjacent bone.
HRCT features.
On high-resolution CT scans of the temporal bones, expansion and erosion of the jugular foramen characterize the glomus jugulare tumor. Perhaps the earliest abnormality detectable on cross-sectional images of a glomus jugulare tumor is erosion of the lateral and anterior walls of the osseous jugular fossa on a thin-sections bone algorithm.
Progressive growth of the tumor produces the typical moth-eaten pattern of erosion of the jugular foramen and destruction of the surrounding bony labyrinth including the caroticojugular spine. The tumor spreads along the paths of least resistance and is initially directed superiorly owing to the intrinsic weakness of this part of the jugular fossa.
Subsequently, the hypotympanum, mesotympanum, and the sinus tympani are invaded. Ossicular chain destruction is common. Inferior spread of the tumor produces infiltration of the IJV and intratemporal fossa. As the tumor spreads laterally, it may destroy the facial nerve canal and infiltrate the facial nerve. On post contrast images the mass shows intense enhancement.
MRI features:
Paragangliomas typically exhibit low signal intensity T1WI and high signal intensity with T2WI. Multiple serpentine and punctate areas of signal void characterize the typical Paraganglioma with all MR sequences; these areas are variably distributed throughout the mass and are believed to represent flow voids in the larger intratumoral vessels.
The classic ‘salt-and-pepper appearance’ was originally described by Olsen et al from appearances on long TR/long TE images. The “pepper” component represents the multiple areas of signal void interspersed with the “salt” component seen as hyperintense foci (due to slow flow or hemorrhage on both short TR and long TR images. This feature is limited to paragangliomas that are greater than 1 cm in diameter and is not considered diagnostic.
In addition to providing superior definition of location, extent, and characterization of paragangliomas, MR imaging also better demonstrates tumor involvement of the ICA and IJV compared with that seen with CT.
MR imaging can depict paragangliomas that are smaller than 5 mm, whereas CT demonstrates only lesions greater than 8 mm
Angiography: It reveals a blush; most often from the ascending pharyngeal artery additionally it may involve the posterior auricular, occipital, maxillary, or internal carotid arteries.
TREATMENT:
Medical therapy: Some cases require no treatment. Often, glomus jugulare tumors are diagnosed within the sixth or seventh decade of life and can be followed by imaging and may not need surgical intervention.
Surgery: It is the treatment of choice for glomus jugulare tumors. Surgical approach depends on the localization and extension of the tumor.
Pre- surgical embolisaion of the tumor can be done.
Radiation therapy and radio-surgery can also be done.
Case of the month – October 2007
Case contributed by Dr. Mukta Agrawal
Clinical profile
A 13-months-old male child presented with gradual distention of abdomen over a period of 25 days with fever and cough since four days. On physical examination, the patient was afebrile and anicteric. There was fullness in the right upper quadrant of the abdomen due to hepatomegaly . No other significant finding was detected on physical examination. Laboratory investigation: SGPT- 22U/L, SGOT-42U/L, Total bilirubin- 0.54mg%, BUN- 11mg%, Hb- 9.3gm%, Serum Alfa fetoprotein- 9.81ng/ml ( normal range- 0 to 13.4 ng/ml), Beta HCG-absent.
Radiological findings
An ultrasound examination of the abdomen (Figs 1,2) revealed a large 10.5 x 9.5 x 10 cm sized anechoic cystic mass occupying almost the entire right lobe of the liver. There were multiple internal septations within. However, no solid component or vascularity within it was seen. The left lobe of the liver was enlarged. The portal vein showed normal flow; its right branch splayed over it anterosuperiorly. No portal or retroperitoneal lymphadenopathy was seen nor was there ascitis. The rest of the abdomino-pelvic ultrasound examination was normal.
Figs. 1, 2
A plain and contrast enhanced CT of the abdomen (Figs. 3,4,5) confirmed the USG findings and showed a large well-defined cystic mass with few internal septations in the right lobe of liver measuring approximately 10 x 8 x 13cms occupying segments VII , VIII and VI. There was no calcification, nor was there any soft tissue component with in. On contrast scan, the septae showed enhancement.
Figs. 3,4,5
The mass was seen to displace the middle hepatic vein; the right hepatic vein was not visualised and the left hepatic vein was normal. The transhepatic IVC was compressed. The mass was abutting the portal vein bifurcation. The main portal vein at the porta and its left branch were normal. The anterior branch of the right portal vein was displaced by the mass forming its anterior margin. The posterior branch of the right portal vein was not seen. The hepatic artery was not hypertrophied. The rest of abdomen was unremarkable. On MRI (figs 6-11), the lesion was hyperintense on T2W image and hypointense on T1W image – conforming its cystic nature. Multiple septa were best seen on T2 W images which demonstrated the complex nature of the cystic mass.
These features suggested a diagnosis of benign mesenchymal hamartoma of the liver.
Discussion:
Incidence:
Mesenchymal hamartomas are uncommon and account for only 8% of all childhood liver masses.
This lesion, though uncommon, is the second most common benign liver tumor of the pediatric age.
Age and presentation: It occurs exclusively during infancy and childhood although few cases in older age groups have been reported. A majority of the cases present at a mean age of 16 months, the range being from newborn to five years of age. The origin is mostly from the right lobe of the liver. There is slight male predominance. Most of the cases remain asymptomatic while others are detected incidentally. The most common clinical presentation is with a right upper quadrant mass, respiratory distress, fever and raised right hemidiaphragm. Occasionally, sudden enlargement may result from rapid fluid accumulation in the cysts. Mass effect from a bulky tumour may cause respiratory distress and lower extremity edema due to compression of the IVC.
Benign liver tumors in children may be divided into two major groups:- those of epithelial derivation, including simple cysts, focal nodular hyperplasia and adenomas and those of mesenchymal derivation including hamartomas and hemangiomas. Benign mesenchymal tumors of the liver are more common than their epithelial counterparts.
A mesenchymal hamartoma is a benign cystic developmental lesion consisting of gelatinous mesenchymal tissue with cyst formation and remnants of normal hepatic parenchyma. It is a large tumor, usually of size 15cm or more at diagnosis, with cysts present in 80% of cases. It is a well-defined tumor that may be encapsulated or pedunculated.
On cut section, this tumor may either have mesenchymal predominance (a solid appearance) or cystic predominance (multiloculated cystic masses). Microscopically, it consists of cysts, remnants of portal traids, hepatocytes, and fluid filled mesenchyme.
Liver function tests usually remain within normal limits. Tumor markers are negative. On CT, mesenchymal hamartomas appear as well-defined masses with central area of low density and internal septations. Both solid and cystic areas are seen. The MR appearances of mesenchymal hamartomas depend on their mesenchymal (stromal) or cystic predominance. Lesions with mesenchymal predominance have lower intensity than normal liver on T2W images because of the fibrotic tissue content. Cystic predominant lesions are of variable intensity on T1W images and are significantly hyperintense on T2W images because of the cystic component. Multiple septae are best seen on T2W images, which demonstrates the complex nature of the cystic mass. Extensive surgery is not necessary because mesenchymal hamartomas are not “true” neoplasms, but a failure of normal development – thus, simple excision, marsupialization, or incisional drainage is all that is required.
Differential diagnosis:
Vascular lesions of mesenchymal origin are hemangioendothelioma and cavernous hemangioma. Hemangioendothelioma usually presents before six months of age and is more common in females. It may be asymptomatic or presents as hepatomegaly, abdominal mass or high output cardiac failure. On CT scan, hypodense well defined homogenous masses are seen with calcification being seen in 40% of cases. Early peripheral enhancement with gradual central filling is evident. Cavernous hemangioma occurs in all age groups and is often asymptomatic and discovered incidentally. It is usually a solitary lesion with predilection for the right lobe and female population. On CT scan, it is seen as a hypodense to isodense well defined mass. On contrast enhancement, early dense, peripheral nodular enhancement with later centripetal fill is noted. In contrast, simple non-parasitic cyst is solitary which may be very large with lining of columnar, cuboidal or flattened epithelium. The wall is thin and composed of mature connective tissue. Simple cysts may also show calcification. Polycystic liver disease on the other hand is frequently associated with polycystic kidneys in about 50% of the patients. Frequently, there is cystic disease of other organs including spleen, pancreas, ovaries and lungs. The cysts may be scattered diffusely or restricted to one lobe – usually the left. Hepatic function is excellent as liver cells are preserved. CT scan is useful in diagnosing the lesion as it does not show enhancement with intravenous contrast. It may remain
asymptomatic – although portal hypertension is common in the infantile variety. Symptomatic patients are usually in the 4th or 5th decade and symptoms are often due to associated polycystic kidneys.
September 2007
Case contributed by Dr. Chetna Wasnik
Case History
A 23-year-old man was admitted with dyspnea on exertion (NYHA grade 3) since one month. There was history of palpitations on and off. Physical examination and laboratory tests were within normal limits.
Investigations:-
A 2D echocardiogram was suggestive of a sinus of Valsalva aneurysm (Figs1A and1B).
1A, 1B
The sinus of Valsalva aneurysm appeared to be partially thrombosed and unruptered and appeared to arise
from the left aortic cusp. Plain and contrast enhanced Cardiac MRI was performed.
Fig. 2A. Haste; axial image (black blood) shows the lumen of the aneurysm as also the thrombosed part.
Fig. 2B TruFisp coronal image (white blood) shows the aneurysm to be arising above the left aortic cusp.
Figs. 3A and 3B Post contrast images axial and coronal.
Patient then underwent a preoperative coronary angiogram. Figs. 4A and 4B show opacification of the aneurysm and its communication.
4A and 4B
DISCUSSION
Thurman first described a Sinus of Valsalva aneurysm (SVA) in 1840. SVA is a rare congenital anomaly accounting for 0.5-3% of all congenital cardiac anomalies. SVA is usually clinically silent but sometimes may present with compression of the adjacent structures or intracardiac shunting caused by rupture of the aneurysm. Approximately 65-85% of SVAs arises from the right sinus of Valsalva followed by the non coronary (10-30%) and left coronary (less than 5%) cusps.
Anatomy:- Sinuses of Valsalva are spaces bounded medially by the aortic valve cusps and laterally by the wall of the aorta. There are three sinuses – right, left and noncoronary. The right coronary sinus is adjacent to the pulmonary tract, the crista ventricularis of right ventricle and the anterior portion of the right ventricle. The left coronary sinus is adjacent to the anterior wall of the left ventricle. The non coronary sinus is adjacent to the right atrium near the atrio-ventricular node and bundle of His.
Age:- Unruptured SVAs are detected insidiously on 2D ECHO even in patients older than 60 years of age. Most ruptured aneurysms are seen in the young individuals in the second or third decades.
Sex:-
Males are affected more than the female. (M: F-4:1).
Presentation:
Approximately one quarter of patients with SVAs are asymptomatic. Dyspnea is the most common presenting symptoms. Rupture of the aneurysm may occur spontaneously or be precipitated by trauma, exertion or cardiac
catheterization. Ruptured SVAs present with specific signs of left to right shunting and they are indistinguishable from coronary arterio-venous fistulas. Those signs are machine type continuous murmurs, bounding pulse, palpable thrill along right or left sternal border.
Ruptured SVAs progress in three stages described by Blackshear.
1) Right chest or right upper quadrant pain
2) Sub acute dyspnea on exertion or at rest
3) Progressive dyspnea, cough, edema and oliguria.
Etiology:-
Primary – Congenital (Idiopathic).
Secondary-
Atherosclerosis
Syphilis.
Cystic medial necrosis; Marfan syndrome.
Blunt or penetrating chest injuries.
Infective endocarditis.
Sinus of Valsalva aneurysms are associated with ventricular septal defect, aortic insufficiency and coarctation of aorta.
Pathophysiology:-
The aneurysm is thought to arise from incomplete fusion of the distal bulbar septum that divides the aorta and the pulmonary artery. It attaches to the annulus fibrous of the aortic valve. It is postulated that after exposure to long standing high pressures, this is the part that forms the sinuses of Valsalva -weakens and becomes aneuysmal.
Imaging:-
1. Plain radiographic features: Since the aortic root is intracardiac, the aneurysm is usually not visible. Rarely, the left aortic sinus aneurysm may bulge in the region of left atrial appendage.
2. Multiplanar transesophageal echocardiography provides precise diagnosis of the aneurysm. It also helps in the identification of the structural anomalies and shunt location for preoperative assessment.
3) 2D ECHO may detect as many as 75% of the SVAs. It may show
– the origin of the sinus
– extension of the sinus.
– associated cardiac conditions.
4) Cardiac MRI-Multiplanar imaging combined with contrast images helps in better delineation of the site and extent of the aneurysm for preoperative assessment.
Treatment:-
Medical management-It usually involves stabilization and preoperative assessment of the patient. Transcatheter closer of the sinus of Valsalva is done by using the Amplatzer device. Surgical treatment-It is mainly for a patient with a ruptured aneurysm.
The surgical procedure includes
– Aortic root reconstructions or replacement.
– Aortic valve replacement of repair.
– Bentall procedure (valve conduit)
– VSD or ASD repair.
– Primary suture closures and patch closures.
Complications:-
1) Myocardial infarction due to coronary artery compression from the aneurysm.
2) Complete heart block due to compression of the conduction tissue by the aneurysm.
3) Right ventricular outflow obstruction.
4) Sudden cardiac death.
5) Infective endocarditis.
6) Tamponade if rupture occurs into the pericardium.
Outcome:-
The prognosis depends upon size of aneurysm and whether it is ruptured or not. Unruptured SVAs need to
be followed up to monitor increase in size. Most of the SVAs increase in the size and rupture. Patients with ruptured SVAs die of heart failure or endocarditis within one year of the onset of symptoms.
November 2006
Case contributed by Dr. Dipak Bankar
CLINICAL PROFILE
A 27-year-old man presented with a swelling on the right side of the neck since one year. The swelling gradually increased in size and had been associated with hoarseness of voice since seven to eight months. The patient also complained of difficulty in deglutition associated with tinnitus in the right ear. Local examination revealed a large cystic mass in the upper lateral part of the right side of the neck with mild pulsatality. Mild loss of the right naso-labial fold was noted. There was right sided mixed hearing loss. On indirect laryngoscopy, there was right vocal cord palsy.
RADIOLOGICAL FINDINGS
A CT SCAN of the base of the skull showed a hypodense lesion (Fig.1) in the region of the carotid fossa. The lesion showed intense enhancement on post contrast images (Fig.2). There was splaying of the ECA and ICA. Another similar lesion was seen in the jugular fossa. On bone windows, there was erosion of the jugular spine (Fig.3).
Fig.1 Fig.2 Fig.3
An MRI of the neck showed a hypointense soft tissue mass in the right jugular fossa. On T1W images, this lesion was seen to extend into the posterior fossa below the level of the external auditory canal. Multiple flow voids were seen within it. The lesion remained hypointense on T2W images as well. The lesion was in contiguity with the carotid canal anteriorly. Another soft tissue intensity mass was seen in the region of the common carotid bifurcation – hyper to isointense on T2W images. This mass produced splaying of the ECA and the ICA (Fig.4) and extended into the parapharyngeal space medially and submandibular region anteriorly. Both the lesions showed intense enhancement on post contrast scans
(Fig.5&6) – showing a classic salt and pepper appearance on post contrast scans.
Fig.4 Fig.5
Fig.6 Fig.7
A FOUR VESSEL ANGIOGRAM was performed. This showed spaying of the carotid bifurcation (Fig.9) and an intense blush (hypervascularity) of the tumour (Fig.10). The characteristic position of the tumour gives the clue to the diagnosis.
FNAC of the lesion was performed. This showed it to be a paraganglioma.
Direct percutaneus glue embolisation of the right carotid body tumour and transarterial sclerotherapy of right glomus tympanicum lesion were performed. There was significant reduction in the vascularity of the lesion. This was shown by the follow up MRI angio (Fig.11) and sonography (Fig.12).
FINAL DIAGNOSIS: GLOMUS JUGULARE AND CAROTID BODY TUMOUR (MULTIPLE
CHEMODECTOMAS).
DISCUSSION
Pathophysiology:
Glomus tumors of the head and neck paraganglia are part of the extra-adrenal neuroendocrine system. At birth, small patches of paraganglionic cells can be widely dispersed throughout the body, mostly in association with autonomic nervous tissue. In the head and neck, such areas include the chemoreceptive areas (glomus tissue) of the carotid bifurcations, the aortic arch, and the temporal bone. The major paraganglia that do not undergo involution are the carotid bodies. They line the medial wall of the bifurcation of the common carotid artery. These paraganglia are functionally important chemoreceptive organ for homeostasis. Specifically, they detect changes in arterial partial pressures of oxygen and carbon dioxide and changes in pH and other blood-borne factors.
Clinical presentation:
Carotid body tumors have no sex predilection. However, studies have shown evidence of a sex predilection with jugulare tumors, with a female-to-male ratios of 5:1. Studies indicate that the incidence of carotid body tumors peaks in patients aged 45-50 years, whereas the incidence of tumors of vagal and jugular origin peaks in those aged 50-60 years. Glomus tumors of the head and neck are extremely rare in pediatric patients.
Patients with carotid body tumors are largely asymptomatic and have a mobile, nontender, slow growing, lateral neck mass. Some patients may report hoarseness and dysphagia associated with compression of the trachea and esophagus and/or vertigo and paresis resulting from cranial nerve compression. Patients with functioning tumors may present with hypertension, headaches, palpitations and tachycardia resulting from increased levels of circulating catecholamines.
Imaging studies
Contrast-enhanced CT demonstrates enhancing soft-tissue masses at characteristic locations – which is a key to the diagnosis. Nonenhanced CT imaging can demonstrate glomus tumors, but the demonstration of a strongly enhancing mass is typical. CT demonstrates carotid body tumors at the level of the carotid bifurcation, respectively splaying the internal and external carotid arteries medially and laterally. These tumors can vary in size, but their location within the bifurcation is critical for diagnosis. Similar to CT imaging, contrast-enhanced MRI demonstrates enhancing soft-tissue masses at characteristic locations. Demonstration of a strongly enhancing mass is typical in the diagnosis of a glomus tumor. As with most
soft tissue tumors, glomus tumors are isointense on T1W MRI and hyperintense on T2W MRI. Contrast- enhanced imaging can show intense tumor enhancement. MRI can show densely enhancing carotid body
tumors at the level of the carotid bifurcation, which respectively splay the internal and external carotid arteries medially and laterally. Sonography can demonstrate the extent of the masses and show their locations. Because of tumor neovascularity, Doppler USG sampling of cervical masses, such as carotid body tumors, can be helpful in diagnosis. Glomus tumors of the head and neck are typically highly vascular, as shown on angiograms. This finding differentiates them from other types of neck neoplasia.
Treatment
The preferred method of treatment for glomus tumors of the head and neck is surgery. However, because most paragangliomas are slow-growing and benign, radiation treatment alone or no treatment at all is preferred in elderly patients in whom the risks of surgery are relatively high and the tumor is unlikely to cause serious morbidity or mortality. If the patient is young, surgery is the best available option because it is the only option that allows total cure.
Embolization is a common technique used as the lone treatment option or as a precursor to surgical excision. As a result of the highly vascular nature of these neoplasms, embolization is an effective technique that is aimed at starving the lesion of its blood supply and inducing necrosis.
Case of the Month – October 2006
Case contributed by Dr. Chirag Khajanchi
CLINICAL PROFILE
A eight-year-old boy presented with a swelling on the posterior aspect of the head since two months associated with headache off and on. The patient had a history of fall during play two years back with resultant torticollis which subsided on its own. On local examination, there was a 8 x 6 cm, ill defined, high occipital swelling with mild pulsatility.
CNS examination was unremarkable with no focal neurological deficit. Routine hematological examinations were normal.
RADIOLOGICAL FINDINGS :
A CT scan of the brain (plain) done two years back at the time of injury was normal.
FIG. 1
A CT scan of the brain at the time of the present admission revealed an expansile, permeative destructive pattern of the parietal bone involving both the inner and outer tables associated with a subgaleal soft tissue swelling. The plain scan revealed a hyperdense mass lesion involving the posterior high parietal region predominantly on the left side with multiple calcifications within. There was associated perilesional white matter edema. Post contrast CT in the early venous phase revealed multiple tortuous vessels suggestive of a vascular malformation. The proximal superior sagittal sinus revealed contrast within it. However, the distal one- third showed no contrast – suggesting thrombosis. Post contrast CT reconstructed in the coronal plane confirmed destruction of the bone with aneurysmal dilatation of the venous channels suggestive of vascular malformations.
FIG.2, FIG.3
FIG.4, FIG.5
MRI study of the brain with contrast showed a large dural AVM at the vertex. Multiple enlarged draining veins were seen. The superior sagittal sinus and the right transverse sinus were enlarged. An underlying solid-cystic soft tissue mass was noted at the vertex. This mass was predominantly on the left side and appeared to be extra axial. It caused destruction of the adjacent bony calvarium. The mass protruded into the scalp through the bony calvarial defect. The mass measured 11.4 x 10.9 x 8.9 cms in maximum craniocaudal, transverse and AP diameters. It was associated with perilesional edema and mass effect. The lesion caused compression of the left lateral ventricle, posterior corpus callosum and brainstem with mild subfalcine herniation to the right side. Subacute intralesional hemorrhage could be seen.
FIG.6, FIG.7
FIG.8, FIG.9
A four vessel angiogram showed a large, highly vascular, dural based mass on the vertex displacing the superior sagittal sinus inferiorly and extending out of the skull. This superior component of the lesion was seen to extend on both the sides of midline. There was another large component of this lesion which was seen inferior to the superior sagittal sinus in the left temporo – parieto – occipital regions. The lesion derived its supply from multiple small branches of both middle meningeal arteries, occipital arteries, dural twigs of dysplastic cortical branches of both middle cerebral arteries, anterior cerebral arteries and posterior cerebral arteries. The draining veins are hypertrophic and dysplastic with multiple ectasias and drained into the hypertrophic superior sagittal sinus.
FIG.10, FIG.11, FIG.12
The child complained of productive cough since two weeks. A chest radiograph revealed a well defined nodular soft tissue mass lesion in the right mid zone. A CT scan of the chest was performed. This showed an isodense nodular mass lesion in the right upper lobe with amorphous calcification and lobulated margins. On post contrast examination, the lesion showed heterogenous enhancement with HU of 35 – 55.
FIG.13
In view of the age of the child and pattern of bone destruction, a diagnosis of Ewings sarcoma was made. The child underwent a biopsy of the skull lesion. The histopathology and immuno histo chemistry confirmed it to be an EWINGS SARCOMA. We believe that the vascularity is related to the tumor though it is difficult to be certain that this is not a separate dural AVM secondary to the trauma.
FINAL DIAGNOSIS : EWINGS SARCOMA OF THE SKULL WITH UNDERLYING DURAL ARTERIO VENOUS FISTULAS WITH LUNG METASTASES.
DISCUSSION
INCIDENCE: Ewing sarcoma is a rare, highly malignant neoplasm of bone accounting for about 5% of biopsied bone tumours. Common sites of primary Ewing’s sarcoma are long bones (47%), pelvis (19%) and ribs (12%). Ewing’s sarcoma of skull vault is rare. It constitutes less than 1% of all the brain tumours. Frontal, parietal and occipital bones are common sites in the skull. Skull base and facial bones are less commonly involved. Seventy five percent of cases are under the age of 20 years, with peak incidence between 5 to 13 years.
PRESENTATION: Usually patients with primary skull vault Ewing’s sarcoma present with pain and swelling. Rarely, the patient may present with a neurosurgical emergency. Neurological signs and symptoms may be present when the tumour is large, compressing or invading the brain parenchyma. These signs and symptoms vary according to size of tumor and region of brain parenchyma involve
HISTOPATHOLOGY: On FNAC, Ewing’s sarcoma shows a cluster of monomorphic tumor cells with round vesicular nuclei and ill defined vacuolated cytoplasm. Many dissociated cells present with naked nuclei. Demonstration of glycogen in the cytoplasm is usually a consistent finding in Ewing’s sarcoma. Histologically, Ewing’s sarcoma is a highly anaplastic tumour with solidly packed small round cells. Light microscopic, ultrastructural and immunohistochemical features differentiate Ewing’s sarcoma from neuroblastoma, lymphoma and rhabdomyosarcoma.
SITES: The most common sites for primary Ewing’s sarcoma is long bones. Diaphysis is more commonly involved, although in 25% cases metaphyses may also be involved. Ewing’s sarcoma in long bones presents with a permeative pattern of medullary destruction a having wide zone of transition, destruction of cortex, multilamellar periosteal reaction and presence of soft tissue.
RADIOLOGICAL FINDINGS
Primary skull lesions present with osteolytic lesion with erosion of inner and outer table associated with soft tissue swelling as seen on plain films.. Similar osteolytic lesions in the skull vault are noted in eosinophilic granuloma; Hand Schuller Christian syndrome, metastasis, Burkitt’s lymphoma, fibrous dysplasia, aneurysmal bone cysts, osteoclastoma and giant cell reparative granuloma. Radiologically it is difficult to distinguish them however, they can be distinguished by different pattern of destruction of skull vault, presence of soft tissue, calcification, cystic component and septations. However histopathology is more specific for their differentiation.
Ewings sarcoma of the skull has a tendency to create a significant epidural mass and push in to brain . The epidural mass is well seen on CT scans .The mass is usually iso to hyperdense on plain scan with heterogenous enhancement on post contrast study. MRI is useful in precise delineation of different tumour components such as extent of bone, dural and parenchymal involvement. Epidural masses rarely invade the dura and invade the brain parenchyma.
Primary Ewing’s sarcoma of the skull has lesser tendency to metastasize to lung and bones (one of few bone tumors metastasizing to bones). From remote primary sites like pelvis and long bones Ewing’s sarcoma may metastasize to skull, spine, meninges and brain parenchyma. Extensive workup is indicated in all cases of Ewing’s sarcoma of skull to search for extracranial primary sites .Radionuclide scanning is most sensitive in early detection of the primary lesion and metastatic deposits. Metastasis to brain parenchyma and dura is less frequent. CNS parenchymal involvement is more common due to primary bone tumour rather than metastatic disease.
Most primary Ewing’s sarcomas have good prognosis because they can be totally or subtotally excised. However, those arising from skull base may involve vital structures prohibiting surgical excision.
TREATMENT:
Surgical removal and /or radiotherapy with adjuvant chemotherapy are treatment of choice. Isolated radiotherapy is indicated in inoperable cases .High risk cases should receive combined radiotherapy and surgical treatment, preferably preoperative irradiation to the lesion
Patients with primary skull vault Ewing’s sarcoma have excellent prognosis with 2-5 years of disease free survival in 50-80% cases.
Case of the month; May 2006
Case contributed by Dr. Alok Sao
Clinical profile
A nine-month-old girl was brought with complaints of recurrent upper respiratory tract infection since the age of two months. She had history of regurgitation after feeds.
Radiological Findings
Plain radiographs of the chest revealed a well-defined soft tissue opacity in the right lower zone in the paracardiac region. Laterally, the margin of the soft tissue opacity could be traced upto the hilum. Inferiorly, the lesion seemed to extend beyond the diaphragm. The thoraco-abdominal sign was positive and the medial border of the opacity could not be well delineated. The right border of heart could be seen well through the soft tissue opacity which was thus interpreted to be posterior placed possibly arising from below the diaphragm. .
Fig.1 Fig.2
A CT scan revealed herniation of the stomach posterior to the heart in the right paraspinal region.
Fig.3 Fig.4
A barium study confirmed the diagnosis of herniation of stomach into the thoracic cavity posterior to the heart on the right side.
Fig.5 Fig.6
Discussion
A congenital diaphragmatic hernia (CDH) is a displacement of abdominal contents into the thoracic cavity through a defect in the diaphragm. Riverius, who incidentally noted a CDH during a postmortem examination of a 24-year-old person, first described CDH in 1679.
CDH occurs 1 in every 2000-4000 live births and accounts for 8% of all major congenital anomalies.
Benjamin et al reported a male preponderance in left-sided hernias – with a male-to-female ratio of 3:2. The incidence is even more striking in right-sided hernias, with a male-to-female ratio of 3:1. The risk of recurrence of isolated CDH for future siblings is approximately 2%.
Age: While CDH is most commonly a disorder of the newborn period, as many as 10% of patients may present after the newborn period and even during adulthood. Outcome in patients with late presentation of CDH is extremely good with low or no mortality.
Pathophysiology:
The three basic types of CDH are the posterolateral Bochdalek hernia (occurring at approximately 6 weeks’ gestation), the anterior Morgagni hernia, and the hiatus hernia. The left-sided Bochdalek hernia occurs in approximately 90% of cases. Left-sided hernias allow herniation of both small and large bowel as well as intra-abdominal solid organs into the thoracic cavity. In right-sided hernias, only the liver and a portion of the large bowel tend to herniate. Bilateral hernias are uncommon and usually fatal. The major problem in a Bochdalek hernia is the posterolateral defect of the diaphragm which results in either the failure of the pleuro-peritoneal folds to develop or the improper or absent migration of the diaphragmatic musculature. Bilateral Bochdalek hernias are rare.
The Morgagni hernia is a less-common CDH, occurring in only 5-10% of cases of CDH. This hernia occurs in the anterior midline through the sternocostal hiatus of the diaphragm, with 90% of cases occurring on the right side.
CDH is characterized by a variable degree of pulmonary hypoplasia associated with a decrease in the cross-sectional area of the pulmonary vasculature and dysfunction of the surfactant system.
Associations: De Lange syndrome, Fryns syndrome, Trisomy 13, Trisomy 18
Clinical presentation:
Infants may have an antenatal history of polyhydramnios. Infants most commonly present with a history of cyanosis and respiratory distress in the first minutes or hours of life – although a later presentation is possible. Frequently, infants exhibit a scaphoid abdomen, respiratory distress and cyanosis. In left-sided posterolateral hernias, auscultation of the lungs reveals poor air entry on the left with a shift of cardiac sounds over the right chest.
Morgagni hernias are usually asymptomatic in the infant.
Imaging :
Antenatal USG- In patients presenting in the prenatal period, ultrasonographic features indicative of CDH include the following: polyhydramnios; an absent or intrathoracic stomach bubble; mediastinal and cardiac shift away from the side of the herniation.
Chest radiograph – Typical findings in left-sided posterolateral CDH include air- or fluid-filled loops of the bowel in the left hemithorax and shift of the cardiac silhouette to the right.
CT scan usually reveals retroperitoneal fat, kidney and bowel loops herniating through the defect.
Barium studies reveals herniation of bowel loops , stomach through the defect.
Treatment :
Until recently, specialists believed that reduction of the herniated viscera and closure of the diaphragmatic defect should be performed emergently following birth. More recent research demonstrates that a delayed surgical approach that enables preoperative stabilization decreases morbidity and mortality. This change is due to the recent understanding that pulmonary hypoplasia, PPHN, and surfactant deficiency are largely responsible for the outcome of CDH and that the severity of these pathophysiologies is largely predetermined in utero. The pathophysiology does not appear to be exacerbated postnatally by herniated viscera in the chest as long as bowel decompression is continuous using a nasogastric tube.
Case of the month April 2006
Case contributed by Dr. Girish Yenvankar
Clinical profile
A 29-yeaar-old lady presented with dysphagia since two years. The dysphagia was more for solids than liquids and was progressively increasing. There was no history of vomiting, weight loss or fever.
Radiological Findings –A plain radiograph of the chest showed a soft tissue opacity in the right paravertebral region with an air-fluid level in the upper dorsal region This suggested a dilated esophagus(Fig 1).
Fig 1
A CT scan of the chest confirmed this. There was narrowing at the lower end of the esophagus. No soft tissue mass was seen in the region of the narrowing (Figs 2,3,4)
Fig 2,3,4
A barium esophagogram a showed smooth narrowing at the gastroesophageal junction extending for a distance of about 2 cms. with moderate dilatation of the proximal esophagus. A bird-beak appearance is seen at the distal portion of the esophagus with no evidence of shouldering. The esophageal mucosal pattern was normal. It showed no intrinsic or extrinsic filling defects or mass effect. Tertiary contractions were seen in the proximal esophagus. There was no evidence of hiatal hernia or gastro-esophageal reflux.
Figs 5,6,7,8
The patient was diagnosed to have achalasia cardia.
Discussion:
Dysphagia is the most common presenting symptom in patients with achalasia. The ingestion of either solids or liquids can result in dysphagia, though dysphagia for solids is more common. The natural history varies. Some patients notice that the dysphagia reaches a certain point of severity and then stops progressing. In others, the dysphagia continues to worsen, resulting in decreased oral intake and malnutrition. Therefore, weight loss is included in the complex of signs and symptoms associated with achalasia, and it is usually a sign of advanced esophageal disease. Some of patients with dysphagia complain of episodes of chest pain which are frequently induced by eating. Typically, chest pain is described as being retrosternal; this is a more common feature in patients with early or so-called vigorous achalasia. As the disease progresses and as the esophageal musculature fails, chest pain tends to abate or disappear. Many of patients with achalasia experience spontaneous regurgitation of undigested food from the esophagus during the course of the disease. Some learn to induce regurgitation to relieve the retrosternal discomfort related to the distended esophagus. As the disease progresses the likelihood that aspiration will occur increases. As a result, some patients may present with signs or symptoms of pneumonia or pneumonitis. Lung abscesses, bronchiectasis, and hemoptysis are some of the more severe pulmonary consequences of achalasia-associated aspiration.
Pathophysiology: The exact etiology of achalasia is not known. The most widely accepted current theories implicate autoimmune disorders, infectious diseases, or both. The last decade has witnessed much progress in the understanding of the cellular and molecular derangements in achalasia. Degeneration of the esophageal myenteric plexus of Auerbach is the primary histologic finding.
Radiologcial Studies –
Plain radiograph -Findings: Plain chest radiographs occasionally offer clues in the diagnosis of achalasia. A double
mediastinal stripe is occasionally depicted. An air-fluid level can be seen in the esophagus; this is frequently retrocardiac. Owing to the paucity of air progressing through the hypertensive LES, the gastric air bubble may be small or absent.
Barium Swallow-
Features of achalasia depicted at barium study under fluoroscopic guidance include the following: Failure of peristalsis to clear the esophagus of barium with the patient in the recumbent position Antegrade and retrograde motion of barium in the esophagus secondary to uncoordinated, nonpropulsive, tertiary contractions Pooling or stasis of barium in the esophagus when the esophagus has become atonic or noncontractile (which occurs late in the course of disease) LES relaxation that is incomplete and not coordinated with esophageal contraction Dilation of the esophageal body, which is typically maximal in the distal esophagus Tapering of the barium column at the unrelaxed LES, resulting in the bird beak sign Associated epiphrenic diverticula sometimes seen.
CT scan –CT scanning with oral contrast enhancement may demonstrate the gross structural esophageal abnormalities associated with achalasia, especially dilatation, which is seen in advanced stages. However, CT findings are nonspecific, and the diagnosis of achalasia cannot be made using CT alone. CT scan may be indicated in the workup of patients with suspected pseudoachalasia.
Treatment: .
Pharmacologic therapy for achalasia: Calcium channel blockers – Nifedipine and verapamil ,Anticholinergic agents – Cimetropium bromide ,Nitrates – Isosorbide dinitrate ,Opioids – Loperamide Pneumatic Balloon Dilatation -Mechanical therapy for achalasia consists of esophageal dilation, the object of which is to disrupt muscle fibers of the LES, effecting a decrease in LES pressure.
Dilation is most commonly performed by using pneumatic balloons.
Botulinum toxin (Botox) therapy –This is new modality of treatment
Esophageal (Heller) myotomy is a surgical procedure that is now commonly performed with minimally invasive techniques. The laparoscopic approach appears to be most appropriate.
July 2007
Case contributed by Dr. Sheetal Mahey
A 25-year-old woman presented with secondary amenorrhea since three years and swelling on the anterior aspect of neck on the right side since one and a half years. Initially, the swelling was peanut sized and increased to the size of an orange within eight months. The swelling was associated with pain and dysphagia which was more for solids than liquids. Subsequently, the patient developed polydypsia and polyuria. For the past eleven months, she had been suffering from low grade fever associated with loss of weight and appetite.
On examination, the general condition of the patient was fair. The pulse was 82/min, blood pressure 120/80 mm Hg. On local examination, there was a diffuse thyroid swelling, soft in consistency on the right side of the anterior aspect of the neck, along with multiple enlarged cervical lymph nodes. No abnormality was detected on examination of the abdomen, respiratory system, central nervous system and cardiovascular system.
Laboratory investigations including hemogram, renal and liver function tests revealed mild elevation of the ESR but were otherwise within normal limits.
Thyroid profile showed reduced TSH levels.
RADIOLOGICAL FINDINGS
The first imaging study performed was a frontal X-Ray of the chest. It revealed reticular nodular opacities predominantly in both lower zones of the lung.
Fig 1
Ultrasonography of the neck was performed using a superficial probe offrequency-12 MHz. This showed heterogeneous enlargement of the thyroid gland. Power Doppler showed increased vascularity within the lesion.
Fig 2
Fig3
A CT scan
of the chest with contrast was performed. This showed patchy centrilobular infiltrates throughout the lung fields with cystic changes best seen in the bases of the lung with evidence of fibrosis and cavitation, sparing the costo-phrenic angles. The apex of lungs was spared.
Fig 4
Fig 5
In view of polydypsia and polyuria, the possibility of diabetes insipidus was considered and an MRI of the brain was performed.
Multiplanar, multiecho MRI of the brain with contrast with 3mm cuts of the sellar region clearly demonstrated a solid intensely enhancing mass measuring 1.0 x 0.9 x 1.0 cm involving the tuber cinerum abutting the posterior aspect of the optic chiasma. The mammilary bodies were seen posterior to the lesion and appeared uninvolved.
Fig 6
Additional T1W axial cuts were taken from the neck region. These showed an enlarged thyroid gland more on the right side with intense enhancement on post contrast study.
Fig 7
A skull radiograph revealed no abnormality.
HISTOPATOLOGY FINDINGS:
An FNAC was performed from the thyroid swelling. This showed plenty of dissociated histiocytic cells, moderate amounts of cytoplasm and vesicular nuclei many of which appeared bilobed and grooved, with deeply clefted nucleus and abundant granular cytoplasm. The background showed lymphocytes, polymorphs and giant cells. No thyroid follicle cell and colloid was seen. Few macrophages and Charcot Layden crystals were seen. Cytomorphology confirmed the diagnosis of Langerhan’s cell histiocytosis.
FINAL DIAGNOSIS:
All imaging and pathological features were consistent with the diagnosis of Langerhan’s cell histiocytosis with CNS involvement (pituitary stalk).
Discussion:
In 1868, Paul Langerhans discovered the epidermal dendritic cells that now bear his name. The term LCH is generally preferred to the older term, histiocytosis X. Langerhans cell histiocytosis or Histiocytosis X is an uncommon disorder. It is characterized by the special type of cells known as Langerhans cells. It is a granulomatous proliferation of the reticular cells at one or several sites in the reticuloendothelial system, possibly related to prior infection.
Histiocytosis is an `umbrella’ designation for a variety of proliferative disorders of histiocytes or macrophages. It encompasses a group of diverse disorders with the common primary event of the accumulation and infiltration of monocytes, macrophages and dendritic cells in the affected tissue. The clinical presentations vary greatly ranging from mild to life threatening.
TYPES:
Histiocytosis is classified into three types.
Langerhans’ cell histiocytosis:
Langerhan’s Cell Histiocytosis – LCH (also known as Histiocytosis X) is a rare disease. Histiocytes are normal cells found throughout the body, in this disease abnormally large numbers are found. LCH is more common in children but it is often seen in adults too.
LCH is divided into two groups:
Langerhans’ cell histiocytosis is sub-divided into three categories:-
(1) Letterer Siwe syndrome which predominantly affects children below five years of age.
(2) Hand Schuller Christian disease which affects children between the age group of 2 and 10
years.
(3) Eosinophillic granuloma affecting children between 5 and 15 years of age.
(4) Hashimoto Pritzker disease (Congenital self healing histiocytosis).
CLINICAL FEATURES:
Clinical manifestations depend on the tissue type affected and to some extent the age – with diffuse severe disease occurring more frequently in younger children than adults. Langerhans cell histiocytosis is an inflammatory disease mostly confined to bone (80%). This can cause pain in the bone and/or swelling on the skull. If the skin is affected, a skin rash, such as cradle cap and nappy rash may occur. A discharge from the ear or hearing problems can occur if the ear is affected. If soft tissue such as lungs, liver, and spleen are affected, then it can lead to difficulty in breathing, jaundice and poor appetite. The hypothalamic pituitary axis may be involved in rare cases causing hormonal problems, this can lead to diabetes insipidus. Occasionally, other pituitary hormones may be affected causing poor growth or delayed puberty. Children over five years of age, often, have only bone involvement. Young children, especially infants, are more likely to have systemic involvement and a fatal outcome.
Causes: The etiology of LCH remains unknown.
Imaging studies:
Plain radiographs:
Radiographically, bone involvement shows multiple lytic lesions and may assume a geographic, permeative or moth-eaten configuration. The borders may be well or poorly defined. In the long bones, endosteal scalloping and medullary expansion are typical. Periosteal reaction is common. In the skull, the edges of the lesion may assume a beveled appearance because of asymmetric destruction of the inner and outer tables of the skull. Most commonly affected bones are skull, mandible, ribs and pelvis. In the mandible, alveolar bone may be destroyed, giving teeth a floating appearance. In the spine, vertebral body involvement usually results in collapse, sometimes to a wafer-thin vertebra plana. As the lesion’s activity begins to wane, periostitis disappears, the margins become well-defined and, reactive sclerosis may develop.
Chest radiographs reveal diffuse cystic changes and reticular opacities throughout the lungs predominantly in the apex of the lungs. However, the lung volumes are normal.
Bases of the lungs are usually spared.
CT-Scan (HRCT):
A high-resolution computed tomography (CT) image demonstrates widespread small, thin-walled lung cysts with resultant parenchymal destruction. All lobes are involved with relative sparing of the costo-phrenic angles. Cysts in the apical segments are relatively thick walled.
In the liver, LCH assumes a nodular appearance. It may also result in periportal fibrosis. LCH in the kidney may cause hydronephrosis from pelvic mass effect. The spleen may be enlarged.
MRI BRAIN:
The pituitary stalk shows thickening and uniform enhancement. The posterior pituitary bright spot is not seen on T1W image in some cases. Cerebral and cerebellar atrophy is often seen. After administration of the contrast agent, there is enhancement of the involved areas.
Most commonly, the hypothalamic-pituitary region is involved, leading to diabetes insipidus (DI). Other involved areas of the brain are cerebellum, basal ganglia, the pons, pineal gland, cerebral hemispheres and ventricles.
DIFFERENTIAL DIAGNOSIS:
Lymphocytic hypophysistis.
Endometritis.
Thyroiditis.
Lymphoma.
AITD with pituitary involvement.
Sarcoidosis.
Tuberculosis.
Idiopathic diabetes insipidus.
Multiple myeloma.
TREATMENT:
Single-system LCH may disappear on its own without any treatment. This may occur even following a biopsy. In some children, treatment such as surgery and corticosteroids may be effective.
Multi-system disease is usually treated with chemotherapy and corticosteroids. The length of treatment varies from child to child.
In our patient, with a history of amenorrhea and diabetes insipidus, all imaging and pathological findings were consistent with the diagnosis of Langerhan’s cell histiocytosis with pituitary stalk involvement
February 2006
Case contributed by Dr.Manish Shinde
A seven-year-old girl presented with complaints of fever and joint pains since one month. She gave history of vague abdominal pain since one month. Since then too, there was a progressively increasing swelling on the vertex of the skull. On examination, this swelling had a firm consistency and was not well defined. The patient’s vital parameters were normal.
RADIOLOGICAL EXAMINATION
A radiograph of the abdomen was unremarkable.
Ultrasonography of the abdomen revealed a well-defined, hypoechoic mass measuring 6x4x4 cms in the left adrenal fossa on the upper pole of the left kidney displacing the kidney inferiorly. It was hypo-vascular with fine calcification within.
Fig 1 Fig2
A plain and contrast enhanced CT scan of the abdomen revealed a well defined 6x4x4 cms hypo dense, hypo vascular mass lesion involving the left adrenal gland with displacement of the kidney inferiorly and laterally. No surrounding infiltration was noted. Fine calcification was seen within the mass.
Fig 3, Fig 4
The skull film showed a destructive lesion with a sunray periosteal reaction over the left parietal bone.
Fig 5, Fig 6
Sonography of the skull confirmed the sunray periosteal reaction and showed a subgaleal hypoechoic soft tissue mass measuring 11x9x 8 cm.
Fig 7
A CT scan of the skull revealed lytic lesions in the left parietal bone with sunray periosteal reaction with subgaleal mass with underlying dural involvement suggestive of a metastatic involvement of the calvarium and dura.
A bone marrow smear was performed which showed atypical round cells suggestive of a neural crest tumour metastases.
MIBG scan showed increased I131 MIBG concentration within marrow cavity of the skull and spine indicating metastases.
Bone scan revealed metastases to the skull and spine.
Final diagnosis – Left neuroblastoma with metastases. Stage 4 INSS staging.
Discussion
Neuroblastomas are the commonest extracranial tumour in children and account for 6-8 % of pediatric malignancies. They originate from the cells of the neural crest origin which give rise to the sympathetic nervous system and adrenal medulla. The median age of patient at the time of diagnosis is two years but the tumour can present at any pediatric age.
Two thirds of patients with neuroblastoma have metastasis (Stage IV disease) at the time of presentation. Metastasis is commonly to the liver, spine, skull and lymph nodes. Staging is done based on the International Neuroblastoma Staging System (INSS) from stage 1 to 4S based on radiological findings, surgical resectability, lymph node involvement and bone marrow involvement.
Technetium 99m methylene diphosphonate whole body bone scintigraphy is a must for detection of metastases. MIBG scan, though less sensitive than MDP should also be done as it can detect both the primary and metastases. FDG PET is likely to play a larger role in neuroblastoma imaging in future.
All patients older than one year with stage IV tumors are considered to be in the high-risk group. These patients seem to require treatment with multi-agent chemotherapy, surgery, and radiotherapy followed by consolidation with high-dose chemotherapy and peripheral blood stem cell rescue.
The five year survival rate from diagnosis is approximately 83% for infants, 55% for children aged 1-5 years, and 40% for children older than 5 years
The residencies start twice a year in February and August.
To apply, you have to collect the forms from the college office about a month prior to the commencement of the residency. These forms are not sent by mail.
The residency program is of three years. As a rule, to be a resident you must have admission to one of the radiology post graduate courses conducted by the University of Mumbai/Maharashtra University of Health Sciences. These include a 2 year diploma course (D.M.R.D.) and a 3 year degree course (M.D.). The details of the number of seats available for these courses is available at http://www.kem.edu/courses.htm . As per the new directives issued by the National Board of Examinations, we are, as a rule, no longer able to provide admissions for the D.N.B. course for those who have completed their MBBS/DMRD courses.
Thus if you are admitted (registered) for the DMRD course, you automatically get a 2 year residency and similarly a 3 year residency is given to those registered for the MD course.
From time to time, depending upon the needs of the department supernumerary third year residents and occasionally fourth year residents are appointed. Those applying for these posts need not be registered for any post graduate courses and may be doing this simply as an additional experience. We do not admit students for The DNB course and no posts will be given on this account.
Depending upon the year of residency, residents are paid between Rs.13,000 and Rs.14,000 per month as stipend.
A lecturer is the junior most staff position. The qualification required is a postgraduate degree (MD,DNB) in diagnostic radiology from a recognized university. Occasionally, if adequate number of candidates are not available, those with a diploma (DMRD) may also be considered for appointment. These posts are filled by the Municipal Medical Service board. The posts are advertised in leading newspapers in Mumbai in April/May each year and the interviews and selection are made in September/October of that year. The number of positions available each year is very variable and is indicated in the newspaper advertisement.
Selection is based on academic qualifications and achievements , experience and performance at the interview.
The salary is between Rs.23,000/- and 28,000/- per month. As a rule quarters are not provided for lecturers.
A postgraduate degree in radiology and 5 years experience as a lecturer qualifies one to apply one for the post of an associate professor. These posts are filled by the Municipal Medical Service board. The posts are advertised in leading newspapers in Mumbai in April/May each year and the interviews and selection are made in September/October of that year. The number of positions available each year is very variable and is indicated in the newspaper advertisement.
Selection is based on academic qualifications and achievements , experience and performance at the interview.
The salary is between Rs.35,000/- and 45,000/- per month. Provision of quarters is subject to availability.
Five years’ experience as an Associate Professor enables one to apply for the post of a professor. The posts are filled by the Maharashtra Public Service Commission and the vacancies are advertised in leading newspapers in Maharashtra from time to time.
Selection is based on academic qualifications and achievements , experience and performance at the interview.
Starting salaries are between Rs. 45,000/- and 55,000/- per month. Provision of quarters is subject to availability.
If you need any specific or more detailed information please send a mail to – websitecontact@kem.edu
| Hemant Deshmukh | Professor and Head |
| Sunita Kale | Professor |
| Padma Badhe | Addl. Professor |
| Priya Hira | Addl. Professor |
| Uday Limaye | Addl. Professor |
| Buz Morris | Addl. Professor |
| Krantikumar Rathod | Addl. Professor |
| Hemangini Thakkar | Addl. Professor |
| Bhavesh Popat | Lecturer |
| Rashmi Saraf | Lecturer |
| Shilpa Sankhe | Lecturer |
| Dev Thakkar | Lecturer |
Introduction :
X rays were discovered at the end of nineteenth century and almost immediately employed in medical diagnosis. Enormous benefits to human health have resulted. The detrimental effects of ionising radiation were recognised early on, with the result that the history of radiation hazards and protection is nearly as long as that of X rays themselves.
Medical irradiation is by far the largest man-made contribution to the radiation burden of the population; for example, a study of the average annual dose to the UK population reveals over 90% of radiation dose from artificial sources is due to medical examination.
Effects of Radiation :
Radiation is fundamentally a random process; it can never be predicted exactly which cells in the body will be effected. However, it would be natural to expect that at high doses more cells might be affected and therefore more damage might be done than at low doses – the severity of the damage and/or the frequency of the damaging events will depend on the dose.
Two terms have been introduced to classify the damage due to radiation through two different processes.
A. Non – Stochastic or Deterministic Effects :
Effects that occur only after a minimum dose has been received and then result in increasing severity of damage and increasing number of transformed cells. Since the word stochastic means random, the term non-stochastic can be taken to mean that effects are not random but quite predictable. To a certain degree the effects can be largely predicted for a particular individuals from the dose received.
Examples of deterministic effects are skin erythema and ulceration.
B. Stochastic Effects :
These effects, in contrast, take place even at very low dose levels, and where the number of cells transforming increases with increasing dose. These are chance events and cannot be predicted accurately in any one individual and can be quantified only in terms of probabilities derived from a study of a large affected populations. Thus the probability of radiation inducing leukemia in a person increases with increasing radiation. Cancer affecting the breast, lung, blood, thyroid and other organs are the main stochastic effects of radiation.
The effect of radiation during pregnancy special consideration since the developing embryo which is subjected to radiation forms a special case of “the innocent patient”.
It would be natural to assume that introducing irradiation into the complicated process of cell development in human fetus would produce some disruption. Special precautions are called for in dealing with a pregnant or potentially pregnant patient, this is because of both the increased risk of radiation damage to the developing fetus and the uncertainties in the clinical situation presented.
Effects for Fetal Irradiation :
Radiation exposure has harmful effects on the development of the fetus. Its severity is explained through the fact that the tissues that form the embryo have only a limited number of cells, so that the death of only a few of these can bring about irreparable damage.
The two principal effects are :
These effects are strongly dependent on three factors :
1. Development stage :
There are three significant periods in embryonic development which exhibit different degree of radio sensitivity. First, a rather short ‘preimplantation stage’ which lasts from fecundation to the fourteenth day (0-14 days). In this stage, the sensitivity of the embryo to radio-exposure is low. The embryo is ,in fact, composed of a population of cells which have not yet been sufficiently differentiated to repair radiation induced. The damaged cells, when in small numbers, are replaced by intact cells coming from new mitosis of the non-injured cells and the embryo develops normally. During this stage, therefore, radiation exposure will have no effect, or if the dose is high, will prevent implantation and the egg will be rejected.
The ‘organogenesis stage’ runs from 15th day to 50th day is characterised by sudden and marked increase in radiosensitivity. In this stage, certain amount of cellular differentiation has emerged. The injured cells, at this stage, differ from one another. A great many malformations and growth deficiencies may be produced during this period, continuing with lesser likelihood into the fetal stage. The particular type of malformation depends upon the organ system differentiated at the time of irradiation. Radiosensitivity is at its maximum between the third and the fourth week of gestation. Radioexposure even at low doses (0.1 Gy i.e.10 Rad) in this period can cause congenital malformation.
At the ‘fetal Stage’ of the development – the stage of simple growth, the embryo is less radio sensitive.
Time Table of Embryo/Fetal Stages :
| Stage |
Times in Days After Conception |
| Pre-implantation |
0-14 |
| Organogenesis |
15-50 |
| Fetal |
51-280 |
Figure 1 illustrates schematically the sensitivity to various adverse effects following a fetal dose of 100 rems delivered at different times during gestation.
Figure 1
Principal Birth Defects :
| ORGAN | KIND OF BIRTH DEFECT |
| BRAIN |
|
| EYE |
|
| SKELETON |
|
2. Radiation Dose :
The probability of malformation and growth retardation falls steeply as the dose is reduced. In its most recent review, the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) has proposed an upper limit of combined radiation risk for several fetal effects (mortality, induction of malformation, mental retardation and childhood cancer) of 3 chances per 100 children for each rem of fetal dose. The estimate for each, of these four effects separately would be somewhat lower. The normal fetal risk for these conditions in absence of radiation was estimated as 60 chances per 1000 children .
3. Radiation Dose Rate:
Reduction of dose-rate generally reduces or eliminates the likelihood of malformation or growth retardation.
Normal Risks for Irradiation in Utero for Absorbed Doses in the Embryo or Fetus :
| Time for Conception | Nominal Risks Per Milligray |
| First two weeks | Minimal |
| 3rd through 8th weeks | Potential for malformation of organs |
| 8th through 15th weeks | Severe mental retardation 1 in 2,500 |
| 16th through 25th weeks | Severe mental retardation 1 in 10,000 |
| Throughout pregnancy | Childhood cancer 1 in 50,000 |
(These nominal risks do not take into account the possible presence of a threshold dose below which severe mental retardation would not occur).
Guidelines for Radiation protection in Pregnancy :
The majority of practicing physicians, at some time in their career, will be faced with a patient who has discovered in retrospect that she was pregnant at a time when extensive x-ray procedures were performed that involved the pelvis or lower abdomen. To make matters worse, as likely as not, the dose will have been delivered during early postconception weeks, because it is unlikely that a pregnancy would remain unsuspected beyond that time and this is the period in which the most disastrous consequences may result from absorption of a given dose of radiation.
Russell, in 1986, introduced the ‘ten day rule’ to prevent untoward effects of radiation in pregnancy. The ‘ten day rule’ states that radiological procedures in women of child bearing age that involve the abdomen or pelvis should only be carried out during first 10 days after a menstrual period of normal duration and intensity. This rule means that x-ray procedures on fertile women who present during the later half of the menstrual cycle should be deferred and an appointment made for after the start of the menstrual cycle to avoid the risk of irradiating an unsuspected concepts. Procedures to be confined to this ‘safe’ period include most nuclear medicine examinations and radiography or fluoroscopy of the abdomen, hip, lumbosacral spine etc.
Elective radiological procedures that should be subject to the 10-day rule include x-ray examinations before employment , annual check ups, and follow up examinations for a previous injury or illness or to monitor the course of a disease, such as a benign breast condition, which warrants ‘regular mammography Xray examinations of this kind, which monitor the patients’ health or existing disease, can certainly be delayed without prejudice until it is definite that the woman is not pregnant.
On other hand, delaying a procedure that is needed to evaluate a new symptom or diagnose a new disease is not justified if the delay could prove detrimental to the health or welfare of the patient. Considering the need for diagnostic test for the mother, the risk to the mother of waiting for the next period, according to the ten-day rule, would be greater than danger to the fetus. This is especially true if conception was found to have occurred, in which case the test should not be carried out al all. These were the considerations behind the 1984 ICRP revision of the rule which now involves no special limitation on exposure in the four weeks following menstruation.
Also in 1984, the National Radiological Protection Board (NRPB) of UK made the following recommendations :
The ICRP in 1984 recommended that a pregnancy should be allowed to proceed if the embryo were exposed to less than 100mGy.
A total dose equivalent limit of 0.5 rem (5mSv) is recommended for the embryo or fetus. Once a pregnancy becomes known, exposure of the embryo or fetus shall be limited to less than 0.05 rem (0.5 mSv) in any month.
Female radiation workers who are pregnant should not exceed the dose limit to the surface of abdomen of 2mSv and a radionucliide intake of 1/20th of the annual limit.
All the means available for reducing doses should be used. These include :
In radiology : high voltages, short exposure times, diaphragm localizer, lead shielding of the abdomen, limitations on the number of films per examination, and number of examinations.
In nuclear medicine : Limitations of the administered activity, choice of radionucliides with short effective half life and pure X-ray emitters.
Procedure to be followed in the case of radio-exposure of the onset of pregnancies :
| Doses in mGy |
Extent of Pregnancy |
||
| Less than Ten Weeks | Greater than Ten Weeks | ||
| Less than 1.5 |
No intervention |
No intervention. | |
| From 1.5 to 15 | Discussion based on the desirability of pregnancy | No intervention | |
| From 15 to 50 | Abortion | No intervention except in the case of complications. | |
| Above 50 |
Abortion. |
Abortion | |
Absorbed radiation doses for typical examinations :
| Examination | Effective Dose (mSv/examination) |
| Simple X-ray : | |
| Lumbar | 2-40 |
| Chest | 0-05 |
| Skull | 0-11 |
| Abdomen | 1-40 |
| Thoracic spine | 0-90 |
| Pelvis | 1-80 |
| Procedures : | |
| IVU | 4-20 |
| Barium meal | 3-40 |
| Barium enema | 7-90 |
| Cholecystography | 0-90 |
| CT (head & body) | 1-10 |
| Nuclear medicine : | |
| 99 m Tc Bone | 5 |
| 99 m Tc Liver | 1 |
| 99 m Tc Brain | 7 |
| 99 m Tc Lung perfusion | 1 |
| 99 m Tc Kidney (DTPA) | 3 |
Flow Chart for Deciding Whether to go in for an X Ray or not :
Flow Chart
The risks of radiation effects on children before birth should be reduced by a policy that balances the necessity and benefit of performing the irradiation with the real risks to the developing child or fetus without causing any compromise of diagnostic study or any delay in diagnosis in mother.
There should be clear local policies on:
Glossary:
Suggested Reading:
The following fellowships awarded by the Maharashra University of Health Sciences is conducted by this department.
See details of the application process here.
| Sub specialty | Duration | Eligibility Criteria | Yearly intake | |
| Radiology | Vascular and Interventional radiology | 1 year | MD or DNB or DMRD in radiology/radio diagnosis | 2 |
| Radiology | Interventional neuro radiology | 1 year | MD or DNB or DMRD in radiology/radio diagnosis2 | 2 |
| Radiology | Body imaging, CT Scan, MRI – Chest and Abdomen | 1 year | MD or DNB or DMRD in radiology/radio diagnosis | 2 |
| Radiology | USG and color Doppler | 1 year | MD or DNB or DMRD in radiology/radio diagnosis2 | 2 |
Radiology
|
Diagnostic neuroradiology | 1 year | MD or DNB or DMRD in radiology/radio diagnosis | 2 |
1-3 months observership is available for all subspecialties in radiology.
Radiologists or radiologists in training are given priority over other physicians for this program:
Qualified radiologists should send in a an application on plain paper with a photocopy of their postgraduate diploma/degree certificate in radiology to the following address: (Residents in training should forward their application through their head of department/institution)
The Dean,
G.S. Medical College and K.E.M. Hospital,
Parel, Mumbai 400 012.
In the application please indicate the number of weeks you would like the obervership to be (minimum 4 , maximum 12 weeks) as also the subspecialty/ies you would like to observe.
You will be notified the acceptance or otherwise of the application along with the allotted time slot.
On the day of the commencement of the course, observers will have to pay a fee at the rate of Rs.1600/- per month for conventional radiology and Rs.3200/- for CT, MRI, Ultrasound, Interventional radiology and such similar courses. For observership for radiographers the charges are Rs.800/- per month. Unfortunately, it is not possible for the institution to arrange for accommodation and observers from outside town are advised to arrange for the same before their arrival in the city.
Besides access to clinical work, observers will be able to make use of the department teaching files and books and electronic media library.
On successful completion of the course, an appropriate certificate will be issued by the institution.
The department provides training for the following courses:
D.M.R.D. Maharashtra University of Health Sciences (MUHS) Two-year course. Five candidates /year
M.D. (Diagnostic Radiology) Maharashtra University of Health Sciences (MUHS). Three-year course (6 candidates /year )
For Details of course kindly visit muhsnashik.com
The following is the approximate distribution of the various postings during a three year residency:
| General radiology | 4 months |
| Gastrointestinal radiology : | 4 months |
| Interventional Neuroradiology : | 1 month |
| Vascular & Interventional radiology : | 3 months |
| Orthopedic radiology : | 1 months |
| Uroradiology : | 2 months |
| M.R.I : | 6 month |
| C.T. : | 6 months |
| Ultrasound : | 6 months |
| Teaching files : | 1 months |
| Pediatric radiology : | 1 month |
| Pre-exam leave : | 1 month |
The Department provide following MUHS approved paramedical courses
B.P.M.T.(Degree Course) Maharshtra university of Health Sciences:Three years course
Ten Candidates/Year
For Details of course kindly visit muhsnashik.com
The following fellowships awarded by the Maharashra University of Health Sciences is conducted by this department.
See details of the application process here.
| Sub specialty | Duration | Eligibility Criteria | Yearly intake | |
| Radiology | Vascular and Interventional radiology | 1 year | MD or DNB or DMRD in radiology/radio diagnosis | 2 |
| Radiology | Interventional neuro radiology | 1 year | MD or DNB or DMRD in radiology/radio diagnosis2 | 2 |
| Radiology | Body imaging, CT Scan, MRI – Chest and Abdomen | 1 year | MD or DNB or DMRD in radiology/radio diagnosis | 2 |
| Radiology | USG and color Doppler | 1 year | MD or DNB or DMRD in radiology/radio diagnosis2 | 2 |
Radiology
|
Diagnostic neuroradiology | 1 year | MD or DNB or DMRD in radiology/radio diagnosis | 2 |
1-3 months observership is available for all subspecialties in radiology.
Radiologists or radiologists in training are given priority over other physicians for this program:
Qualified radiologists should send in a an application on plain paper with a photocopy of their postgraduate diploma/degree certificate in radiology to the following address: (Residents in training should forward their application through their head of department/institution)
The Dean,
G.S. Medical College and K.E.M. Hospital,
Parel, Mumbai 400 012.
In the application please indicate the number of weeks you would like the obervership to be (minimum 4 , maximum 12 weeks) as also the subspecialty/ies you would like to observe.
You will be notified the acceptance or otherwise of the application along with the allotted time slot.
On the day of the commencement of the course, observers will have to pay a fee at the rate of Rs.1600/- per month for conventional radiology and Rs.3200/- for CT, MRI, Ultrasound, Interventional radiology and such similar courses. For observership for radiographers the charges are Rs.800/- per month. Unfortunately, it is not possible for the institution to arrange for accommodation and observers from outside town are advised to arrange for the same before their arrival in the city.
Besides access to clinical work, observers will be able to make use of the department teaching files and books and electronic media library.
On successful completion of the course, an appropriate certificate will be issued by the institution.
The department provides training for the following courses:
D.M.R.D. Maharashtra University of Health Sciences (MUHS) Two-year course. Five candidates /year
M.D. (Diagnostic Radiology) Maharashtra University of Health Sciences (MUHS). Three-year course (6 candidates /year )
For Details of course kindly visit muhsnashik.com
The following is the approximate distribution of the various postings during a three year residency:
| General radiology | 4 months |
| Gastrointestinal radiology : | 4 months |
| Interventional Neuroradiology : | 1 month |
| Vascular & Interventional radiology : | 3 months |
| Orthopedic radiology : | 1 months |
| Uroradiology : | 2 months |
| M.R.I : | 6 month |
| C.T. : | 6 months |
| Ultrasound : | 6 months |
| Teaching files : | 1 months |
| Pediatric radiology : | 1 month |
| Pre-exam leave : | 1 month |
The Department provide following MUHS approved paramedical courses
B.P.M.T.(Degree Course) Maharshtra university of Health Sciences:Three years course
Ten Candidates/Year
For Details of course kindly visit muhsnashik.com
The information on these pages is meant only to be a general guide for patients. To ensure simplicity and avoid confusion, several medical details have been excluded from this discussion, Thus, these are not meant to substitute interaction with your doctors. Different doctors have different ways of investigating patients and you should always follow the instructions of your doctors whenever you are asked to undergo any investigations.
Please bear in mind that whenever you go for a radiological examination, you should carry with you all relevant medical information including reports of all previous investigations. Please make it a point to carry with you all previous radiological reports as this may help your radiologist to arrive at a diagnosis for your present problem.
As practiced today, x-ray examinations are very safe. With improvement in the quality of the equipment used and radiation protection standards, no patient (with the exceptions described below) should avoid having an radiological examination for fear of harm by radiation.
There are however, a few situations where x-ray examinations may be more harmful.
For example – in young patients – whether they are boys or girls, additional protective measures will be taken by your radiologist to protect their reproductive organs.
X ray examinations performed during pregnancy may have adverse effects on the unborn child. This includes malformations of the fetus and increasing chances of childhood leukemia. These risks are maximal in the first three months of pregnancy and decrease substantially during late pregnancy. Therefore, in pregnancy, an x-ray examination will not be advised unless it is absolutely essential. If you are pregnant or likely to be pregnant, you should inform you doctor of this. Your radiologist will always ask for this and will advise of the pros and cons of the test that you have been asked to undergo. In case you have been accidentally exposed to radiation during pregnancy, and wish to know what is to be done (continue or terminate the pregnancy) please consult your radiologist. (You could also write to us with all details and we may be able to advise you on the best course of action).
Radiation produced by CT scans is the same as for x rays and rules are essentially the same as mentioned above for x rays.
MRI and Ultrasound examinations are, for all practical purposes, safe at all times and should not be a cause of concern even during pregnancy.
These are substances that are used by the radiologist during the course of certain radiological investigations to obtain clearer view of the various structures in the body. These contrast media which are popularly known as “dyes” are of various types and are used in standard radiological examinations, in CT scans and during MRI,
The following paragraph may sound a little technical,, but as it has important practical information on these injections, please read it carefully.
The iodine based contrast agents that we were talking about earlier, are broadly divided into two categories. The older “Ionic” variety and the newer “Non ionic” variety. The main difference is that the incidence of adverse reactions is vastly less with the newer “Non ionic” agents as compared to the older “Ionic” agents. The only reason the newer and safer “Non ionic” contrast agents are not universally used is that they are up to five times more expensive than the older “ionic” contrast agents. In case you suffer from any of the conditions mentioned above or even otherwise, if you do not want to take any risks, you could ask your radiologist to use the newer ” non ionic” medium- provided you are willing to pay additional cost for the same. Please note that even in the United States, the routine use of the newer: “Non ionic” contrast medium is not recommended as it is not felt to be cost effective in view of the relatively low incidence of adverse reactions even with the older “ionic” contrast injections.
Also, even with the newer “non ionic” contrast agents adverse reactions can occur but only very rarely.
These are investigations performed using barium to evaluate the digestive tract (the gullet, the stomach, the small and large intestines). For all practical purposes, there are no side effects of these procedures and in most cases, you can resume normal activity and resume normal diet immediately after these investigations.
Barium swallow ( also known as an esophagogram) – for the evaluation of the esophagus (gullet)
Barium Stomach duodenum (SD) also known as the “Upper GI series” – for the investigation of the stomach and the upper part of small intestine.
Barium follow through (FT) also known as the “small bowel series” – for evaluating the small intestine.
Small bowel enema Selective and specialized visualization of small intestine
Barium enema for investigating the large intestine.
Barium swallow
This is a short, painless procedure which takes 10-15 minutes to perform. You may be asked to starve overnight for the procedure. You will be asked to swallow the barium liquid several times as the radiologists observes its flow under fluoroscopy (x ray screening). He will also take x rays as required in different positions. In some cases an intravenous injection may be given to relax the muscles of the esophagus to obtain good quality pictures.
You will get the final reports on the evening of the examination or on the following day.
Barium stomach-duodenum
This procedure is also similar to the barium swallow. You will be asked to report on empty stomach after overnight starvation. It is painless and takes up to 20 -30 minutes to perform, In addition to the barium; you will be asked to swallow a gas producing powder which will give better quality x ray pictures. In some cases an intravenous injection may be given to relax the muscles of the stomach to obtain good quality pictures. X ray pictures will be taken in different positions to visualize the various parts of the stomach and the duodenum.
You will get the final reports on the evening of the examination or on the following day.
Barium follow through
In addition to overnight starvation and reporting on empty stomach, often you will be asked to take laxative tablets on the night before the examination. When you report for the examination, you will be given one or more glassfuls of barium to drink and x ray pictures will be taken at periodic interval (hourly or so). Towards the end, your abdomen may be observed under fluoroscopy (screening) and more pictures taken. At this time, a enema tube may be passed and air instilled into your large intestine (this part of the procedure tends to be a little unpleasant though is essentially painless. You may even feel a very strong urge to pass stools , but you will have to control it for a few minutes).
The whole examination may take anywhere between an hour and 4- 6 hours (typically about 2 hours) depending on how soon the barium passes through the small intestine. Though not painful, the waiting may be a little boring. In some cases you may be asked to report for a “24 hour” film on the following morning. (At this time no further barium is given nor do you have to come starving) Pictures of the left over barium in the intestines is taken. You will get the final reports on the evening of the examination or on the following day.
Small bowel enema
When detailed information on the small intestine is required and especially when it is not available even after the barium follow through examination described above, you may be advised to undergo a small bowel enema. For this procedure again, you will have to report on empty stomach after overnight starvation. You may be asked to take laxative tablets on the night preceding the examination to clean your bowels.
For this procedure a long rubber tube is passed through your mouth (or sometimes through the nostril) and is guided by the radiologist into the small bowel under screening control. This part of the procedure takes about 10 minutes and can be unpleasant – especially when the tube is being passed through the gullet. In spite of this, the procedure is essentially painless. Once the tube is in place, the radiologist will inject barium and some other liquid through this tube ( when this is done , you will feel nothing) and x ray pictures of your small intestine are obtained. The whole procedure lasts about 30 -45 minutes.
You will get the final reports on the evening of the examination or on the following day.
Barium enema
This examination is performed for the visualization of the large intestine. For two or more days before the procedure, you will be asked to take purgative tablets to clean the large intestine. Some radiologists insist that on the day prior to the investigation, the patient take only light food or sometimes only liquids. All this is quite inconvenient but is essential to the performance of a good study and the more you cooperate the better the quality of the examination. On the morning of the procedure, most radiologists will allow their patients to drink fluid.
For the procedure itself, a thin suspension of barium is prepared and is then introduced into the large bowel through a enema tube. Following this air is pushed through the tube with the help of an pump. During this time, there is no real pain but patients experience varying degrees of discomfort and urge to pass stools.
Serial x-ray pictures are obtained under fluoroscopy to depict the various parts of the large intestine.
After the procedure, you will be allowed to pass stools and can resume a normal diet immediately.
The formal report is usually available on the day following the examination.
IVP stands for intravenous pyelography. This is also variously known as IVU (intravenous urogram or simply a urogram).
This procedure is done to evaluate the kidney, the ureter (the tubes that connect the kidney to the urinary bladder) and the urinary bladder
It is essential to have a clear picture of the abdomen before this procedure is done. Therefore most radiologists will prescribe that the patient take laxative tablets for two nights prior to the procedure. Also tablets to reduce the gas in the abdomen are also prescribed for 2-3 days prior to the investigation. This may be quite inconvenient but is essential to the performance of a good study and the more you cooperate the better the quality of the examination. You will be asked to starve overnight and report on empty stomach.
At first a “plain” x-ray of the abdomen is obtained. Amongst other things, this checks on how clean the abdomen is of feces and gas. Sometimes, if there is excessive feces or gas, the procedure may not be done and a further day’s abdomen cleansing tablets may be recommended.
Once the plain x ray film of the abdomen is seen to be satisfactory; a intravenous iodinated contrast injection will be give. This is the only painful part of this study.
After the injection, 8-10 x rays of your abdomen will be obtained for up to 1-2 hours. After the procedure is over, you can take normal diet and resume normal activities immediately.
Reports are usually available on the day following the examination.
This procedure is almost always performed in men to study the urinary bladder and the urethra (the passage through which urine is passed).
Patients may be asked to refrain from drinking water for several hours before the procedure. A catheter (a thin hollow tube) is passed through the urethra into the urinary bladder. This is done under local anesthesia and may be mildly painful. Then the urinary bladder is filled with contrast medium and x ray films are obtained. One the films may be done while the patient is actually passing urine on a special x-ray table.
The procedure lasts about 30 minutes and the patient can resume normal activities immediately after the procedure. Sometimes, a course of oral antibiotics may be prescribed by the radiologist.
This procedure is done in males to look for narrowing of the urethra (the passage through which urine is passed).. No prior preparation is required. Iodinated contrast injection is made into the urethra after application of local anesthesia and X rays are obtained at this time. The procedure lasts around 10 minutes. No significant pain or only mild pain is associated with this procedure. You can resume normal activity immediately after the procedure. Reports are usually available the day following the procedure.
Ultrasonographic examinations can be performed for virtually every part of the body; though are not very useful for evaluation of diseases of the brain and chest. They are most useful for diseases of the abdomen, during pregnancy and for evaluation of blood vessels (color Doppler).
In general all ultrasonographic examinations are painless; at most may cause mild discomfort and have no adverse effects. It is generally agreed that with ultrasonography, there is no danger to the unborn child. at any stage of pregnancy.
Except for ultrasonography of the abdomen, where prior preparation is required, no special preparation is essential for other ultrasonographic examinations. In some cases, patients may be instructed to drink plenty of water and not to pass urine for a couple of hours prior to ultrasonography of the abdomen.
Depending upon the type of examination, ultrasonography may take from 15 minutes to up to a hour especially if color Doppler studies are to be done.
Color Doppler is similar to ultrasonography, where the radiologist will evaluate diseases of the blood vessels with the use of ultrasonography.
This procedure can be used to diagnose abnormalities of virtually any part of the body. This technique also uses x-rays to visualize various structures in then body, but in most areas, provides vastly more information than available from x ray films. The radiation safety concerns that apply to simple x-rays are the same for CT scans too.
Being essentially painless, the procedure needs no anesthesia; however, in cases of children and patients who are unable follow instructions, sedation or a short anesthesia may occasionally be necessary.
The procedure is essentially painless. However, for many CT scan examinations, intravenous injections of iodine based contrast is made and this could be a source of minor pain or discomfort. Also, in some cases, (especially abdominal scans), patients may be asked in addition to drink about two glasses (reasonably palatable) contrast medium before the commencement of the scan.
For plain CT scans (scans which do not need intravenous contrast injection), no preparation is required and these can be done at any time. If a contrast medium is to be injected, patients are usually asked to starve for 4-6 hors prior to the procedure.
The time required for most CT scans varies between 10 minutes and 45 minutes, and most of the time, all that the patient is expected to do is to lie as still as possible and follow simple breathing commands.
After the scan is over, the patient can resume normal activities almost immediately.
Depending upon the urgency of the procedure, reports can be made available within minutes of the completion of the examination. Routine reports are usually available by the following day.
These are variations of normal CT scans ; but these procedures are seldom performed these days and are generally indicated only if MRI scans cannot be done for some reason.
And are indicated for diagnosis of the compression of nerve roots and spinal cord or for cases of cerebrospinal fluid (CSF) leak. For both these procedures, non-ionic, iodinated contrast medium is injected by a lumbar puncture. A lumbar puncture is done by inserting a needle under local anesthesia between the bones in the low back. It is a moderately painful procedure and may take up to 15 minutes. The patient has to starve for about 4 hours prior to the procedure. After the lumbar puncture is done, the contrast is injected into the fluid casing around the spinal cord. Once this is done, the patient will be examined in the scanner for about 15-30 minutes. After the scan is over, the patient will have to take complete bed rest for up to 24 hours. Many patients experience varying degrees of headaches for a day or two after this investigation. In spite of the pain and discomfort, the procedure is, by and large, safe.
MRI most useful for investigating various disorders of the brain, the spinal cord, blood vessels, muscles and joints, the heart and blood vessels
This is a type of radiological examinations that do not use x-rays and hence there are no radiation “hazards”. For all practical purposes, the procedure is painless there are no complications with the procedure. However, if have had any metallic implants done in you at a time of a previous surgery (such as pace maker implants, nails and other hardware used by orthopedic surgeons, brain aneurysm clip etc); please bring this to the attention of the radiologist. He/she will then decide if it is safe for you to undergo this examination.
The equipment is similar to CT scanner in appearance; however, while lying inside the machine, some patients may feel claustrophobic and the examination may not be possible. For these patients there are the newer “open magnet” systems where there is no fear of claustrophobia.
Sometimes, the radiologist may inject contrast intravenously during the course of the MRI study. This often aids in the diagnosis.
As a rule no pre procedure preparation is required. Generally, the procedure lasts between 30 minutes and an hour and you will have to lie still during this type and follow simple breathing commands. The procedure being painless, no anesthesia is required, however, in case of small children and other patients who are unable to lie still, sedation or a short anesthesia may be required.
You can resume normal activities immediately after the procedure.
Depending upon the urgency of the procedure, reports can be made available within minutes of the completion of the examination. Routine reports are usually available by the following day.
This is a procedure which is seldom performed these days. It is use to investigate symptoms arising out of compression of nerves and the spinal cord with in the spinal column.
By and large, this procedure has been replaced by MRI and CT scans.
For this procedure, non ionic, iodinated contrast medium is injected by a lumbar puncture. A lumbar puncture is done by inserting a needle under local anesthesia between the bones in the low back. It is a moderately painful procedure and may take up to 15 minutes. The patient has to starve for about 4 hours prior to the procedure. After the lumbar puncture is done, the contrast is injected into the fluid casing the spinal cord. Once this is done, the patient will be examined on an x ray table in varying positions (including a “head low position”) This takes an additions 15-30 minutes.. After the procedure is over, the patient will have to take complete bed rest for up to 24 hours. Many patients experience varying degrees of headaches and backache for a day or two after this investigation. In spite of the pain and discomfort, the procedure is, by and large, safe.
Reports are available on the day following the procedure.
This procedure includes the study of blood vessels (arteries and veins) in the body.When, as is the most common case, arteries are studied it is called an arteriogram, (most of the time, the word angiogram is used synonymously with an arteriogram) and when the veins are studied it is called a venogram.Depending upon which particular artery is examined, these procedures have special names such as carotid angiograms – when vessels of the brain are investigated; coronary angiogram when the arteries supplying the heart are studied or a renal angiogram when the arteries of the kidney are looked at.In the current practice of angiography, small tubes called catheters ( they are about the diameter of a ball pen refill) and introduced into various blood vessels thru a small puncture of the artery in the groin. Sometimes, the entry point may be through blood vessels the upper limb (axilla, elbow or wrist), Through this tube, iodinated medium is injected and x rays are obtained. These x rays show up the state of your blood vessels.The following is typically what will happen when are to undergo an angiogram:A few days before the angiogram, blood tests may be done to make sure that the clotting parameters f your blood are normal. and that your kidneys are functioning normally.To avoid infection, hair in and around your groins will have to be shaved before the procedure. You will be asked to get admitted in a hospital on the night before or on the morning of the angiogram and will be expected to take nothing by mouth for 4-6 hours before the procedure.
In most situations, angiograms are done under local anesthesia. However, in cases of small children or adults who are unable to cooperate or in altered sate of consciousness, the procedure may be done under deep sedation or even general anesthesia.The moment you enter an angiogram room, you will see a bewildering array of equipment. There will also a be 5-6 persons including nurses, technicians, orderlies and of course the radiologist. Do not get intimidated by all this. To avoid infection, those performing the angiogram wall be dressed in as in operation theater. You will be asked to lie down on the x-ray table and an intravenous saline infusion may be started. Then a nurse wills cleanup your groin area with an antiseptic agent.The radiologist will then anesthetize the groin area a through which the angiogram is to be done (mostly on the right side; sometimes the left and occasionally both sides).The local anesthetic is injected with a very fine needle and the pain of this injection is just like any other ordinary injection you may have taken. After this, you will feel very little or no pain when the angiogram is being done. The radiologist will then puncture the blood vessel in the groin (remember – this will be essentially painless or mildly painful) and insert the catheter into the blood vessels – none of this procedure incites any pain. He will then put the catheter under x ray control into the desired blood vessel ( this is also painless) and inject some contrast medium and take x rays. During the actual injection of the contrast medium, for 5-10 seconds, you will feel varying degrees of warmth in the area being injected and rarely even mild pain. If non ionic contrast media are injected, there is no warmth or pain. During all this you will hear a lot of equipment noise, lots of talks between those present in the room (pay no heed to all this) and the table on which you are lying may be move up and down, sideways etcAn average angiogram lasts between 30 and 90 minutes.After the angiogram is finished, the catheter will be taken out and pressure will be applied on the puncture site to stop bleeding. This will last 5-10 minutes and is mildly painful. You will then be sent back to your hospital room. You should not move from bed or move the limb through which the angiogram has been done for at least 6 hours. Some radiologists may recommend bed rest for up to 24 hours. During this time, if there is any bleeding from the puncture site, do not get alarmed – apply firm pressure to stop the bleeding and ask for help. You will be allowed to take fluids after about 2 hours of the angiogram and then solids a couple of hors later. Reports will be available the following day.Thus an angiogram is a minor procedure, entails hospitalisation for 12-24 hours, and is mildly painful and generally risk-free.A venogram is a much simpler procedure, usually done without catheterization,. Normally it is done for lower limb varicose veins and an intravenous injection of contrast medium is made in the foot and multiple x rays are obtained. The procedure is minimally painful. The procedure lasts about 15 minutes and you can resume your normal activities immediately thereafter.
Angioplasty ,Stenting ,Embolisation:
Angioplasty is a procedure in which blocked blood vessels are opened up using catheters; so is stenting. Embolisation is the opposite – here abnormal or leaking blood vessels are closed with substances introduced through catheters. These and similar procedures where the radiologist treats diseases of blood vessels and other organs is known as interventional radiology and are generally performed as an alternative to surgery or in some cases as the only form of treatment where no surgery is possible.These are procedures which are similar to an angiogram in most respects. The main difference is – whereas an angiogram is used to diagnose blood vessel disease , angioplasty and embolisation are used to treat diseases of blood vessels or the organ that these blood vessels supply (brain, kidney, liver etc). The procedure of catheterization for both angioplasty , stenting and embolisation is similar to that of angiography. However, depending upon the specific area being treated and the specific condition for which the procedure is done, there will be variation in the technique, procedure time and complications. As the indications and the scope of these procedures is very vast; it is very difficult to generalize. If you have any specific questions, please send your queries to us.
This procedure is done to visualize the female reproductive system, i.e.: uterus & Fallopian tubes. The indication is usually infertility.
The procedure is usually done on the 9th or 10th day after the onset of menses (when the chances that the patient may have conceived are the least).
The procedure is done without anesthesia; some patients may need some pain killers during the procedure as some may experience moderate pain.
Patients are instructed to shave their private parts (pubic hair) & are asked to bring one of their relatives along with them at the time of investigation.
The procedure involves cannulation of the cervix under direct vision with a cervical cannula & injection of radio-opaque contrast media into the uterus. Then multiple x-rays are taken to record the flow of contrast into the uterus and the tubes.
The procedure lasts about 30 minutes; patients may have some pain for a few hours after the procedure; but usually resume normal activities immediately thereafter. Patients may be asked to take a course of oral antibiotics after the procedure
The report will be available on the evening of or the morning after the procedure.
In a few cases of excessive watering of the eyes, a Dacryocystogram may be advised by the ophthalmologist.
This investigation is done to check the patency of the nasolacrimal duct, which is a small tubular passage which drains the tears from the eyes into the nose.
No preparation is required for the procedure. A small cannula (blunt needle) is introduced into a normally present hole on the lower eyelid. When this is done, there is no or very little pain or discomfort. Through this cannula, contrast medium is injected. There will be no pain when this injection is made; often there is a salty taste in the mouth as the contrast injection normally flows to the back of the mouth.
At this time one or more x ray films of the eye are taken.
The patient can resume normal activities as soon as the procedure is over. The average procedure time is around 15 minutes. The report is available on the following
Most patients we examine are those that are being treated at the K.E.M. hospital
or at other hospitals run by the Mumbai Municipal Corporation or other state run
hospitals. The cost of radiological examinations for these patients is as follows:
Plain films Rs. 30 per film
Barium and IVP Rs. 200
Angiograms cost Rs.2500 – Rs.5000
CT scans(plain and or contrast) Rs.1200
MRI scans are Rs. 2500 (plain and or contrast)
Ultrasonograms at Rs.100 and Color Doppler studies at Rs. 500
In case of emergent or poor patients, these charges are waived.
However, patients who are referred to this department from private practitioners
or hospitals for specialised tests or treatment procedures will be entertained by
our department on a case to case basis depending upon the medical needs.
These patients are charged for these diagnostic or therapeutic procedures – the
charges being variable depending upon the consumables used. For details please
contact the department office on 02224107536
Peripheral vascular disease, or P.V.D, is a common yet serious disease that affects people as they age, and increasing the risk of Heart attack and Stroke. P.V.D. (or Bad Circulation) is a condition in which the arteries that carry blood to the arms or legs become narrow due to deposition of fatty material in their walls. When the deposits of fatty material reduces the blood flow significantly, then the limbs start aching on walking. Such aching subsides on taking rest. The most common cause of P.V.D is atherosclerosis (often called hardening of the arteries). Atherosclerosis is a gradual process in which fat, cholesterol and other substances accumulate in the walls of the arteries forming a substance called “plaque” that clogs the blood vessels.
Since atherosclerosis is a generalized disorder, P.V.D. is a collator for all diseases caused by the obstruction of large Blood Vessels in the body. It may not be confined to one artery but may involve arteries in other areas of the body as well. Some of the more commonly affected areas are the arteries of the kidneys (Renals), neck (Carotids) and the most important being the HEART. Stroke, Heart attack and gangrene of legs are the most obvious consequence of such damaged arteries.
Timely detection and treatment of P.V.D. can improve your quality of life, and help reduce your risk of having Stroke, leg amputation and even death.
Symptoms of P.V.D:
Risk Factors for P.V.D:
Those who are at highest risk are:
A family history of heart or Cardio- vascular disease may also put you at higher risk for P.V.D. Remember that if you have P.V.D. than the risk of Heart Attack and Stroke is higher.
The most common screening test for P.V.D. is the Ankle-Brachial Index (ABI) , a painless test in which a special stethoscope is used to compare the blood pressures in your feet and arms.
In some cases, lifestyle changes are enough to halt the progress of P.V.D. and manage the disease. Sometimes, medications or procedures that open up clogged blood vessels are given to treat PVD.
Exercise
Regular exercise is the most effective treatment for P.V.D.
Regular aerobic exercise like walking at a medium pace for 30 to 40 minutes, three to four times a week provides optimal benefits for the legs as well as for the heart.
Some people may have a medical condition that prevents them from participating in an exercise program. Consult your physician before undertaking any exercise or other treatment program.
Diet
Limit intake of saturated fat, for example, meat, egg –yolk, dairy products, coconut oil, yogurt, coffee, salt, fried junk food and sugars (chocolates and pastries).
It is advisable to eat high fiber foods such as vegetables, fresh fruits and whole grains. Oat bran, apples, olive oil, almonds, millet (ragi) and rice –bran oil (RBO – The Indian Olive oil) all help reduce cholesterol levels. In fact, RBO is also supposed to have significant cholesterol-and triglyceride-reducing properties, The cholesterol reducing property is attributed to the presence of a unique anti-oxidant, oryzanol, which is not found in any other edible oil. Try to include them in your diet everyday. Strict dietary discipline can lower cholesterol levels by upto 10%.
Stop Smoking
There is no doubt that cigarette smoking is a strong risk factor for P.V.D. and heart disease. On an average, smokers are diagnosed with PVD as much as 10 years earlier than non-smokers. Stopping smoking now is the single most important thing you can do to halt the progression of P.V.D. or prevent it in the future. In fact, stopping smoking reduces your risk of even heart attack within a year and renders you equal to a non-smoker in three years.
Medications
Vitamin B Complex, especially B3 and B6 are essential for fat metabolism and help support the liver, a hard working organ that processes fat. Vitamin E (400 IU daily) helps circulation, magnesium and potassium regulate heart spasms. Vitamin C reduces bad cholesterol in the blood. Good dietary sources of B vitamins include dark-green leafy vegetables, citrus fruits and juices, dried beans, nuts, oatmeal, wheat germ, and yeast.
Aspirin and other blood-thinning agents may be taken under medical supervision to improve blood circulation.
Drugs that lower cholesterol (Statins) or control high blood pressure may be prescribed. New medications that help prevent blood clots or the build up of plaque in the arteries, or that reduces the pain of P.V.D., are also appropriate for some patients.
Angioplasty — In this procedure, an Endovascular Specialist inserts a very small balloon attached to a thin tube (catheter) into a blood vessel through a small puncture in the skin at the groin. The catheter is threaded under X-ray guidance across the site of the narrow or blocked artery. The balloon is then inflated to open the artery.
Stents — In some cases, a tiny spring-like cylinder made of biocompatible metal mesh called stent, is inserted in the clogged vessel to act like scaffolding and keep the artery widely open after Angioplasty.
Thrombolytic therapy — An Endovascular Specialist uses this treatment if the blockage in the artery is caused by a blood clot. Thrombolytic drugs—sometimes called “clot busters”—dissolve the clot and restore blood flow. Usually, the drugs are administered through a spray directly into the clot. This treatment is sometimes combined with another treatment, such as angioplasty if the clot is too rigid.
Stent-grafts — a Stent covered with synthetic fabric is inserted into the blood vessels to bypass diseased arteries.
Surgical Treatments for P.V.D.
Thrombectomy. This procedure is used only when symptoms of P.V.D. develop suddenly as a result of a blood clot. In the technique, a balloon catheter is inserted into the affected artery beyond the clot. The balloon is inflated and pulled back, bringing the clot with it. Thrombectomy is a small surgical procedure.
Bypass grafts. In this procedure, a vein graft from another part of the body or a graft made from artificial material is used to create a detour around a blocked artery.
The Best Treatment for PVD:
The best treatment for PVD depends on a number of factors, including your overall health, the location of the affected artery, and the size and cause of the blockage or narrowing in the artery.
You should discuss all your treatment options with your Endovascular Specialist.
Dr. Krantikumar Rathod
(Patients are cautioned that this information is for general use only. In case you have queries or need further details, please contact us at the telephone numbers given at the end of this note)
Fibroids (also called as leiomyomas) are benign (noncancerous) swellings of the muscle wall of the womb (uterus). These are not uncommon in women in the reproductive age group. They occur as single or multiple masses & vary in size enormously – from that of grapes to that of pumpkins!. Though some fibroids (especially the small ones) may produce no symptoms, many can cause several problems depending on their size and position in the womb. Some women with fibroids have heavy, prolonged and painful bleeding during periods sometimes leading to anemia (deficiency of blood). This may also be associated with problems in the patient becoming pregnant or can lead to recurrent abortions. They can give rise to increase in the frequency or inability to pass urine, particularly before periods. Sometimes, they can cause constipation. Most women with fibroids also complain of heaviness in the lower abdomen.
The cause of fibroids is not known but their growth is dependant upon the estrogen hormone in the body. During pregnancy & with oral contraceptives, when there is excess of estrogen in the body, fibroids are known to grow rapidly; whereas, after menopause (termination of menstrual life), when there is much less estrogen in the body, fibroids are known to shrink, although larger fibroids may persist. New fibroids rarely appear after menopause.
Uterine fibroids, which are small and not causing problems do not require treatment. Many women go through their lives having fibroids without being aware of them and having no gynaecological problems .If symptomatic & treatment is required, it is important to be aware of the available treatment options.
Conventional treatments for fibroids
Organ sparing options:
Medicines:
They do not cure fibroids but may relieve symptoms associated with fibroids.
Pain killing medicines:
If fibroids cause pain, pain-killing tablets may be given. These are usually anti-inflammatory drugs such as NSAIDs ( e.g. Brufen).
Iron therapy:
For anemia due to excessive bleeding.
Progesterone hormones:
If periods are heavy, progesterones are given to reduce the amount of blood loss. However, as the underlying problem is not a hormone imbalance, this treatment is not usually effective.
GnRHa analogue:
These are synthetic drugs which act like hormones that are produced by body. They have the effect of reducing the level of estrogen. This in turn results in the reduction in blood flow to the uterus and the fibroid. This hormone drug can reduce the size of fibroids temporarily. However, as soon as drug is stopped, the fibroids grow back to their original size within few months. This drug can only be given for six months, as after this, it can cause thinning of bones. It is mainly used to reduce the size of fibroids, before surgery to make operation easier.
Uterus preserving surgery-Myomectomy:
This is a major operation usually done in infertile women or women desirous of childbearing and wishing to retain the uterus. This requires a stay in the hospital of five to six days and four to six weeks off work. This involves removing of the fibroids out of the uterus but leaving the uterus in place. However, as there are often several fibroids, it is rarely possible to remove all of them, as this would cause too much damage to the womb. The advantage of this procedure is that, as the uterus is left behind, it is possible to become pregnant. There are two possible disadvantages. Firstly, this surgery can leave behind adhesions (scarring) inside the pelvis, which, at worst can cause the fallopian tubes to become blocked. This would then prevent pregnancy from occurring. Secondly, on rare occasions, when myomectomy is being carried out, there may be bleeding to such an extent that it becomes necessary to remove uterus (hysterectomy) as an emergency measure to stop the bleeding.
Non-organ sparing options:
Hysterectomy: (Removal of the uterus)
This is the most effective treatment of fibroid as there is no possibility that the fibroids can regrow afterwards. This surgery also requires a stay in hospital of five to six days and four to six weeks off work, depending on nature of work. The major disadvantage of this operation is that women can no longer become pregnant & therefore is not suitable for people who have not completed their family.
Uterine Artery Embolisation (UAE) in the treatment of uterine fibroids
This is new organ sparing treatment for uterine fibroids that does not require an operation. UAE is a new way of treating fibroids by blocking off the arteries – uterine arteries – that carry blood to the fibroids, making the fibroid shrink. This procedure is performed by an interventional radiologist, a physician especially trained in to perform this procedure with specialized embolisation experience. This was first reported in 1995 by group in France. Since then, it has been widely practicised all over the world and proved to be a viable alternative treatment for uterine fibroids. This is an effective, minimally invasive procedure with relatively less pain, less complications and shorter duration of hospital stay and lay off from work.
Procedure
The procedure is done after consultation with the gynecologist. Pre-procedure imaging in the form of ultrasonography or MRI of pelvis is required to assess the response after the procedure with follow up imaging. This procedure is done in the radiology department in a special screening room called as DSA (digital subtraction angiography) suite. The patient needs to be admitted in the hospital a day prior to the procedure .As the procedure is done using an artery in the groin, the patient is asked to shave the skin around the groin. The patient needs to be fasting for four hours prior to procedure.
During the procedure, the patient lies down on the DSA table. A needle is put in arm vein to give sedative or pain killer. General anesthesia is not required. Monitoring devices are attached to chest or fingers to monitor vital parameters. The skin over the groin is prepared with antiseptics & rest of the body is covered with sterile towels.
There is no need for general anesthesia and the patient is fully awake during the procedure. The skin & deeper tissues over the artery in the groin area are anesthetized with local anesthetic, (once this given patient do not feel pain) and then needle is inserted into artery. Once this is correctly placed, a guide wire is passed into artery over which a device called sheath is inserted. Through the sheath, a fine plastic tube called catheter is passed into arteries feeding fibroids, which are called right and left uterine arteries under x-ray control. All this is painless. A radiological dye called contrast medium is injected into these arteries, which may cause a warm feeling in pelvis. Once the fibroid’s blood supply has been identified, the catheter is navigated into the uterine arteries ( one on each side ) & fluids containing small particles are injected into these arteries,which blocks these arteries so that fibroids are starved of their blood supply. Both the right and left uterine arteries are blocked in this way .At the end of the procedure, the catheter and sheath are withdrawn and interventional radiologist compresses the puncture site for about ten minutes to prevent any bleeding. Six-hour immobilisation of leg in which the puncture is made is required. The times taken for complete procedure vary depending how complex and straightforward the arterial anatomy in a particular patient is procedure is, but generally it is between 1-2 hours. The patient is kept for monitoring & antibiotic medication in the ward for two days. The patient can join her regular work three to four days following UAE.
Fibroid embolisation is normally a safe procedure but there are some risks and complications that can arise as with any medical treatment. Occasionally, a small bruise called hematoma around the site where the needle has been inserted can occur, which is quite normal. Most patients feel pain, fever , particularly in first twelve hours but can be controlled by painkillers. A few patients may get vaginal discharge persisting for approximately two weeks.. Infection is the most serious complication of fibroid embolisation .The signs of this include pain, pelvic tenderness and high fever. Post procedure antibiotic prophylaxis is given to prevent the same.
Uterine artery embolisation is well-tolerated, safe and effective procedure in the treatment of fibroids, and has opened a new door to success for patients in whom other treatments are ineffective or contraindicated.
If you require further information regarding UAE,
please contact
Dr.Hemant Deshmukh /Dr.Krantikumar Rathod on Tel. 24107000 ext 2543 or 24103906 or email us on websitecontact@kem.edu
The following two patients who have undergone UAE and have agreed to talk to any patient who wants to know a patient’s perspective can be contacted at the following addresses.

Mrs.Anusaya Shamrao Desai
“I was suffering from pain in the lower abdomen and heavy periods since three years I consulted the gynecology OPD at K.E.M. Hospital. My sonography done at K.E.M.Hospital showed a large fibroid in the uterus which was causing heavy periods & pain in the lower abdomen. The doctor advised me to undergo immediate operation to remove the uterus. On hearing the word operation, I was frightened & worried. Since I am diabetic & hypertensive, I was high risk for surgery. Then the doctor suggested me the option of uterine artery embolization for which I was referred to the vascular & interventional radiology section. There doctor explained me the procedure which gave me confidence. Accordingly, I underwent this procedure in this department which was tolerable with uneventful recovery. After one month, I got my sonography done which showed decrease in the size of the fibroid. I never had heavy periods following this procedure. Thanks to doctors and Hospital authorities”.
Room No.6,
Tata Mills Chawl No. 6/C,
J.B.Road,
Parel,
Mumbai-400012

Mrs.Madhuwanti Suhas Bhagwat
It was vicious circle.
Due to heavy loss of blood, the hemoglobin level was always low. Iron deficiency anemia was persistent and this would again lead to heavy periods causing heavy loss of blood for more than five days.
The hematologist had confirmed that it was iron deficiency anemia and need for immediate treatment of mennorhagia. Pelvic sonography revealed multiple intramural uterine fibroids. The gynecologist had recommended hystrectomy .I had my reservation about hystrectomy as it would lead to blood loss during surgery, post operative care, might lead to harmone replacement therapy and so on…
My friend who herself had undergone this procedure and benefited immensely suggested the option of uterine artery embolisation. I met Dr.Hemant Deshmukh and Dr. Krantikumar Rathod in last week of September 2002 .The doctors were positive about results and benefits I would get after the UAE procedure.
I underwent this procedure on 4th October 2002. I could interact with doctors during the procedure & there was no pain at all. I started my routine work within one week.
Mrs.Madhuwanti Suhas Bhagwat
Granthalaya Services E -2/3:1,
Sector 1, Vashi
Navi Mumbai-400703
E-mail: bmadhuwanti@rediffmail.com
Phone: 27823719 / 27826546
Introduction :
X rays were discovered at the end of nineteenth century and almost immediately employed in medical diagnosis. Enormous benefits to human health have resulted. The detrimental effects of ionising radiation were recognised early on, with the result that the history of radiation hazards and protection is nearly as long as that of X rays themselves.
Medical irradiation is by far the largest man-made contribution to the radiation burden of the population; for example, a study of the average annual dose to the UK population reveals over 90% of radiation dose from artificial sources is due to medical examination.
Effects of Radiation :
Radiation is fundamentally a random process; it can never be predicted exactly which cells in the body will be effected. However, it would be natural to expect that at high doses more cells might be affected and therefore more damage might be done than at low doses – the severity of the damage and/or the frequency of the damaging events will depend on the dose.
Two terms have been introduced to classify the damage due to radiation through two different processes.
A. Non – Stochastic or Deterministic Effects :
Effects that occur only after a minimum dose has been received and then result in increasing severity of damage and increasing number of transformed cells. Since the word stochastic means random, the term non-stochastic can be taken to mean that effects are not random but quite predictable. To a certain degree the effects can be largely predicted for a particular individuals from the dose received.
Examples of deterministic effects are skin erythema and ulceration.
B. Stochastic Effects :
These effects, in contrast, take place even at very low dose levels, and where the number of cells transforming increases with increasing dose. These are chance events and cannot be predicted accurately in any one individual and can be quantified only in terms of probabilities derived from a study of a large affected populations. Thus the probability of radiation inducing leukemia in a person increases with increasing radiation. Cancer affecting the breast, lung, blood, thyroid and other organs are the main stochastic effects of radiation.
The effect of radiation during pregnancy special consideration since the developing embryo which is subjected to radiation forms a special case of “the innocent patient”.
It would be natural to assume that introducing irradiation into the complicated process of cell development in human fetus would produce some disruption. Special precautions are called for in dealing with a pregnant or potentially pregnant patient, this is because of both the increased risk of radiation damage to the developing fetus and the uncertainties in the clinical situation presented.
Effects for Fetal Irradiation :
Radiation exposure has harmful effects on the development of the fetus. Its severity is explained through the fact that the tissues that form the embryo have only a limited number of cells, so that the death of only a few of these can bring about irreparable damage.
The two principal effects are :
These effects are strongly dependent on three factors :
1. Development stage :
There are three significant periods in embryonic development which exhibit different degree of radio sensitivity. First, a rather short ‘preimplantation stage’ which lasts from fecundation to the fourteenth day (0-14 days). In this stage, the sensitivity of the embryo to radio-exposure is low. The embryo is ,in fact, composed of a population of cells which have not yet been sufficiently differentiated to repair radiation induced. The damaged cells, when in small numbers, are replaced by intact cells coming from new mitosis of the non-injured cells and the embryo develops normally. During this stage, therefore, radiation exposure will have no effect, or if the dose is high, will prevent implantation and the egg will be rejected.
The ‘organogenesis stage’ runs from 15th day to 50th day is characterised by sudden and marked increase in radiosensitivity. In this stage, certain amount of cellular differentiation has emerged. The injured cells, at this stage, differ from one another. A great many malformations and growth deficiencies may be produced during this period, continuing with lesser likelihood into the fetal stage. The particular type of malformation depends upon the organ system differentiated at the time of irradiation. Radiosensitivity is at its maximum between the third and the fourth week of gestation. Radioexposure even at low doses (0.1 Gy i.e.10 Rad) in this period can cause congenital malformation.
At the ‘fetal Stage’ of the development – the stage of simple growth, the embryo is less radio sensitive.
Time Table of Embryo/Fetal Stages :
| Stage |
Times in Days After Conception |
| Pre-implantation |
0-14 |
| Organogenesis |
15-50 |
| Fetal |
51-280 |
Figure 1 illustrates schematically the sensitivity to various adverse effects following a fetal dose of 100 rems delivered at different times during gestation.

Figure 1
Principal Birth Defects :
| ORGAN | KIND OF BIRTH DEFECT |
| BRAIN |
|
| EYE |
|
| SKELETON |
|
2. Radiation Dose :
The probability of malformation and growth retardation falls steeply as the dose is reduced. In its most recent review, the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) has proposed an upper limit of combined radiation risk for several fetal effects (mortality, induction of malformation, mental retardation and childhood cancer) of 3 chances per 100 children for each rem of fetal dose. The estimate for each, of these four effects separately would be somewhat lower. The normal fetal risk for these conditions in absence of radiation was estimated as 60 chances per 1000 children .
3. Radiation Dose Rate:
Reduction of dose-rate generally reduces or eliminates the likelihood of malformation or growth retardation.
Normal Risks for Irradiation in Utero for Absorbed Doses in the Embryo or Fetus :
| Time for Conception | Nominal Risks Per Milligray |
| First two weeks | Minimal |
| 3rd through 8th weeks | Potential for malformation of organs |
| 8th through 15th weeks | Severe mental retardation 1 in 2,500 |
| 16th through 25th weeks | Severe mental retardation 1 in 10,000 |
| Throughout pregnancy | Childhood cancer 1 in 50,000 |
(These nominal risks do not take into account the possible presence of a threshold dose below which severe mental retardation would not occur).
Guidelines for Radiation protection in Pregnancy :
The majority of practicing physicians, at some time in their career, will be faced with a patient who has discovered in retrospect that she was pregnant at a time when extensive x-ray procedures were performed that involved the pelvis or lower abdomen. To make matters worse, as likely as not, the dose will have been delivered during early postconception weeks, because it is unlikely that a pregnancy would remain unsuspected beyond that time and this is the period in which the most disastrous consequences may result from absorption of a given dose of radiation.
Russell, in 1986, introduced the ‘ten day rule’ to prevent untoward effects of radiation in pregnancy. The ‘ten day rule’ states that radiological procedures in women of child bearing age that involve the abdomen or pelvis should only be carried out during first 10 days after a menstrual period of normal duration and intensity. This rule means that x-ray procedures on fertile women who present during the later half of the menstrual cycle should be deferred and an appointment made for after the start of the menstrual cycle to avoid the risk of irradiating an unsuspected concepts. Procedures to be confined to this ‘safe’ period include most nuclear medicine examinations and radiography or fluoroscopy of the abdomen, hip, lumbosacral spine etc.
Elective radiological procedures that should be subject to the 10-day rule include x-ray examinations before employment , annual check ups, and follow up examinations for a previous injury or illness or to monitor the course of a disease, such as a benign breast condition, which warrants ‘regular mammography Xray examinations of this kind, which monitor the patients’ health or existing disease, can certainly be delayed without prejudice until it is definite that the woman is not pregnant.
On other hand, delaying a procedure that is needed to evaluate a new symptom or diagnose a new disease is not justified if the delay could prove detrimental to the health or welfare of the patient. Considering the need for diagnostic test for the mother, the risk to the mother of waiting for the next period, according to the ten-day rule, would be greater than danger to the fetus. This is especially true if conception was found to have occurred, in which case the test should not be carried out al all. These were the considerations behind the 1984 ICRP revision of the rule which now involves no special limitation on exposure in the four weeks following menstruation.
Also in 1984, the National Radiological Protection Board (NRPB) of UK made the following recommendations :
The ICRP in 1984 recommended that a pregnancy should be allowed to proceed if the embryo were exposed to less than 100mGy.
A total dose equivalent limit of 0.5 rem (5mSv) is recommended for the embryo or fetus. Once a pregnancy becomes known, exposure of the embryo or fetus shall be limited to less than 0.05 rem (0.5 mSv) in any month.
Female radiation workers who are pregnant should not exceed the dose limit to the surface of abdomen of 2mSv and a radionucliide intake of 1/20th of the annual limit.
All the means available for reducing doses should be used. These include :
In radiology : high voltages, short exposure times, diaphragm localizer, lead shielding of the abdomen, limitations on the number of films per examination, and number of examinations.
In nuclear medicine : Limitations of the administered activity, choice of radionucliides with short effective half life and pure X-ray emitters.
Procedure to be followed in the case of radio-exposure of the onset of pregnancies :
| Doses in mGy |
Extent of Pregnancy |
||
| Less than Ten Weeks | Greater than Ten Weeks | ||
| Less than 1.5 |
No intervention |
No intervention. | |
| From 1.5 to 15 | Discussion based on the desirability of pregnancy | No intervention | |
| From 15 to 50 | Abortion | No intervention except in the case of complications. | |
| Above 50 |
Abortion. |
Abortion | |
Absorbed radiation doses for typical examinations :
| Examination | Effective Dose (mSv/examination) |
| Simple X-ray : | |
| Lumbar | 2-40 |
| Chest | 0-05 |
| Skull | 0-11 |
| Abdomen | 1-40 |
| Thoracic spine | 0-90 |
| Pelvis | 1-80 |
| Procedures : | |
| IVU | 4-20 |
| Barium meal | 3-40 |
| Barium enema | 7-90 |
| Cholecystography | 0-90 |
| CT (head & body) | 1-10 |
| Nuclear medicine : | |
| 99 m Tc Bone | 5 |
| 99 m Tc Liver | 1 |
| 99 m Tc Brain | 7 |
| 99 m Tc Lung perfusion | 1 |
| 99 m Tc Kidney (DTPA) | 3 |
Flow Chart for Deciding Whether to go in for an X Ray or not :

Flow Chart
The risks of radiation effects on children before birth should be reduced by a policy that balances the necessity and benefit of performing the irradiation with the real risks to the developing child or fetus without causing any compromise of diagnostic study or any delay in diagnosis in mother.
There should be clear local policies on:
Glossary:
Suggested Reading:
A Website with comprehensive information for patients
The Department of Radiology at the Seth GS Medical College and King Edward Memorial (KEM) hospital, Mumbai is the pioneering department of Interventional Radiology in India and has been in the forefront of image guided interventions (general vascular, non vascular and neurovinterventions) in the country.
The cases on this page, represent a sampling of the wide variety of Interventional radiological procedures practiced in the department. From time to time, we will be adding new cases to this page.
We hope these cases will be use both to physicians wanting to refer patients and those patients who are looking for a high volume center to treat their problems by interventional radiological techniques.
College Central Library :
1.Abdominal Imaging
2.American Journal of Roentgenology (AJR)
3.Cardiovascular and Interventional Radiology
4.Journal of Clinical Ultrasound
5.Radiographics
6.Radiologic Clinics of North America
7.Radiology
Department Library :
1. American Journal of Roentgenology (Electronic) – Courtesy ARRS
2. Asian-Oceanic Journal of Radiology
3. Clinical Radiology (Courtesy – Anil ‘Adi” Ahuja , Hong Kong)
4. Current Problems in Diagnostic Radiology
5. Journal of Computer Assisted Tomography (Courtesy – Anil “Adi” Ahuja – Hong Kong)
6. Journal of Medical Ethics
7. Journal of Postgraduate Medicine
8. Journal of Ultrasound in Medicine
9. Journal of Vascular and Interventional Radiology
10.Indian Journal of Radiology and Imaging
11.Interventional Neuroradiology (Courtesy Prof. Marco Leonardi)
12.MR Imaging Clinics of North America
13.Neuroimaging Clinics of North America
14.Radiographics (Electronic) Courtesy RSNA
15.Radiologic Clinics of North America (Courtesy – Pramod Phadke, Australia)
16.Radiology (Electronic) – Courtesy RSNA
17.Revista Neuroradiologica (Courtesy Prof. Marco Leonardi)
We request our Alumni to help us subscribe to more journals. If you wish to , please write to websitecontact@kem.edu
King Edward Memorial Hospital and Seth Gordhandas Sunderdas Medical College
Acharya Donde Marg, Parel, Mumbai 400 012. India.